Oxidized CaMKII causes cardiac sinus node dysfunction in mice - PubMed (original) (raw)
. 2011 Aug;121(8):3277-88.
doi: 10.1172/JCI57833. Epub 2011 Jul 25.
Anil Purohit, Siddarth Soni, Niels Voigt, Madhu V Singh, Alexey V Glukhov, Zhan Gao, B Julie He, Elizabeth D Luczak, Mei-ling A Joiner, William Kutschke, Jinying Yang, J Kevin Donahue, Robert M Weiss, Isabella M Grumbach, Masahiro Ogawa, Peng-Sheng Chen, Igor Efimov, Dobromir Dobrev, Peter J Mohler, Thomas J Hund, Mark E Anderson
Affiliations
- PMID: 21785215
- PMCID: PMC3223923
- DOI: 10.1172/JCI57833
Oxidized CaMKII causes cardiac sinus node dysfunction in mice
Paari Dominic Swaminathan et al. J Clin Invest. 2011 Aug.
Abstract
Sinus node dysfunction (SND) is a major public health problem that is associated with sudden cardiac death and requires surgical implantation of artificial pacemakers. However, little is known about the molecular and cellular mechanisms that cause SND. Most SND occurs in the setting of heart failure and hypertension, conditions that are marked by elevated circulating angiotensin II (Ang II) and increased oxidant stress. Here, we show that oxidized calmodulin kinase II (ox-CaMKII) is a biomarker for SND in patients and dogs and a disease determinant in mice. In wild-type mice, Ang II infusion caused sinoatrial nodal (SAN) cell oxidation by activating NADPH oxidase, leading to increased ox-CaMKII, SAN cell apoptosis, and SND. p47-/- mice lacking functional NADPH oxidase and mice with myocardial or SAN-targeted CaMKII inhibition were highly resistant to SAN apoptosis and SND, suggesting that ox-CaMKII-triggered SAN cell death contributed to SND. We developed a computational model of the sinoatrial node that showed that a loss of SAN cells below a critical threshold caused SND by preventing normal impulse formation and propagation. These data provide novel molecular and mechanistic information to understand SND and suggest that targeted CaMKII inhibition may be useful for preventing SND in high-risk patients.
Figures
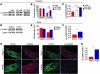
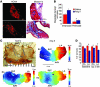
Figure 1. ox-CaMKII is increased in heart failure patients and in Ang II–infused mice.
(A) Representative immunoblots showing ox-CaMKII and total CaMKII from right atrial tissue obtained from heart failure patients with SND requiring pacemaker (HF+PM) implantation, heart failure patients without SND (HF–PM), and controls (–PM). The lanes were run on the same gel but were noncontiguous. (B) Summary data for ox-CaMKII and total CaMKII in HF+PM (n = 5), HF–PM (n = 6), and –PM (n = 10) patients (*P < 0.05, #P < 0.01). (C) Increased ox-CaMKII normalized to total CaMKII from patient samples shown in B (*P < 0.05 and #P < 0.01). (D) Representative immunoblots showing ox-CaMKII and total CaMKII from right atrial tissue obtained from WT mice infused with Ang II or saline for 3 weeks. The lanes were run on the same gel but were noncontiguous. (E) Summary data for ox-CaMKII and total CaMKII in Ang II– (n = 6) and saline-infused (n = 6) mice (*P = 0.03). (F) Increased ox-CaMKII normalized to total CaMKII in mouse samples shown in E (*P = 0.02). (G) Representative immunofluorescence images showing that 3 weeks of Ang II infusion increases SAN ox-CaMKII. SAN area is identified by HCN4 immunostaining (green). Scale bars: 50 μm. (H) Summary data showing increased ox-CaMKII normalized to total CaMKII in SAN tissue from mice treated with 3 weeks of Ang II or saline infusion (n = 4/group, *P < 0.01).
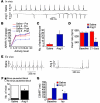
Figure 2. Ang II infusion causes SND.
(A–D) In vivo data from unanesthetized and unrestrained ECG-telemetered mice. (E–H) Ex vivo data from Langendorff-perfused mouse hearts. (A) A representative ECG recording of spontaneous bradycardia in an Ang II–infused mouse and a normal ECG recording in a saline-infused mouse. (B) Diminished spontaneous activity-responsive HR (ARHR) (P < 0.01) in mice after 3 weeks of Ang II infusion compared with other groups (5 mice/group). Pre, prior to mini-osmotic pump insertion; Post, 3 weeks after infusion. (C) Summary data showing a trend (P = 0.06, n = 4–5/group) toward reduced episodes where HR was less than 200 beats/min in Ang II– compared with saline-infused mice. (D) Ang II infusion for 3 weeks reduced resting in vivo HR, compared with 3 weeks of saline infusion (n = 25/group, P < 0.001). (E) Representative ECG recordings from Langendorff-perfused hearts isolated from mice infused with Ang II or saline for 3 weeks. (F) Summary data showing that Ang II–infused mice have more sinus pauses than saline-infused mice (*P = 0.023, n = 11/group). (G) Hearts isolated from Ang II–infused mice have prolonged CSNRT compared with saline-infused controls (*P = 0.04, n = 10). Iso, isoproterenol.
Figure 3. Myocardial and SAN-targeted CaMKII inhibition protects against SND.
(A) ECG-telemetered, unrestrained, and unanesthetized AC3-I transgenic mice (n = 8–10/group) infused with Ang II for 3 weeks have no significant decrease in resting HR or (B) reduction in ARHR or (C) increased bradycardia events. *P < 0.01 compared with baseline; †P < 0.01 compared with all other groups. (D) Ex vivo Langendorff-perfused AC3-I hearts isolated after 3 weeks of Ang II infusion have no significant increase in sinus pauses (n = 8/group). (E) Gene painting with an adenovirus-poloxamer mixture (see Methods) allowed for SAN-targeted expression of IRES eGFP from the CaMKIIN adenovirus (scale bars: 25 μm). (F) WT mice (n = 5–6/group) with SAN expression of CaMKIIN and eGFP were significantly (*P = 0.02) resistant to reduced resting HR after 3 weeks of Ang II infusion compared with WT mice with SAN eGFP expression alone. (G) Langendorff-perfused hearts from WT mice with SAN-targeted expression of CaMKIIN and eGFP were significantly (*P = 0.049, n = 5–6/group) protected against Ang II–induced increases in sinus pauses compared with WT mice expressing eGFP alone. (H) SAN-targeted CaMKIIN expression significantly protected against Ang II infusion induced CSNRT prolongation in response to 80-ms (*P = 0.04) and 100-ms (#P = 0.049) pacing cycle intervals. The same hearts were studied in G and H.
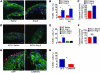
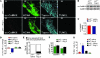
Figure 4. Ang II infusion causes SAN cell death and SND.
(A) Representative SAN tissue section showing increased TUNEL staining (red) in mice infused with Ang II compared with saline-infused mice. Blue staining shows nuclei (DAPI), and green shows HCN4; scale bars: 20 μm for all micrograph panels. (B) Summary data showing increased TUNEL-positive cells in the SAN (intranodal, *P = 0.001, n = 5/group) and in SAN-adjacent tissue (perinodal, #P = 0.006, n = 5/group) and (C) activated caspase-3 compared with saline-infused controls (†P < 0.001, n = 7/group). (D) Representative SAN tissue sections showing that Ang II infusion for 3 weeks does not increase TUNEL staining in AC3-I mice. (E) Summary data showing that the AC3-I mice infused with Ang II for 3 weeks are resistant to SAN cell death (P = 0.89 for AC3-I Ang II versus saline and *P = 0.001 for WT Ang II versus saline, n = 5/group) and (F) caspase-3 activation (P = 0.73) compared with saline-infused controls. (G) Representative tissue sections showing that SAN-targeted eGFP and CaMKIIN expression protects against Ang II–induced TUNEL staining compared with SAN-targeted eGFP expression alone in WT mice. (H) Summary data from studies in G (*P = 0.001, n = 6/group).
Figure 5. A structurally based mathematical model of the SAN.
(A) Structural model of 2-dimensional tissue slice with color-coded representation of different regions, including atrial cell, peripheral SAN, and central SAN zones. CT, crista terminalis. (B) Experimentally measured and simulated percentage of apoptotic cells in WT and AC3-I SAN following infusion with saline or Ang II. (C) Simulated activation cycle length of right atrial (RA) free wall during sinus rhythm in the WT and AC3-I tissue model following 3 weeks of infusion with saline or Ang II. Ang II infusion slows RA activation in the WT but not in the AC3-I model. Exp, experimental; Sim, simulated. (D) SAN diastolic depolarization rate (DDR) from the central SAN region in the WT and AC3-I models following saline or Ang II infusion. Ang II infusion slows SAN DDR in the WT but not in the AC3-I model. (E and F) Simulated central SAN (top) and right atrial (bottom) action potentials from WT (E) saline- and (F) Ang II–treated models. The decrease in functional SAN tissue mass due to Ang II infusion slows spontaneous SAN firing rate and RA activation rate. Episodes of transient SAN exit block are observed in the WT Ang II–treated model as evidenced by a SAN action potential (red asterisk) that fails to propagate into the RA free wall region, resulting in an activation pause (red arrow). Vm, cell membrane potential.
Figure 6. SAN and atrial fibrosis are promoted by Ang II and contribute to SND.
(A) Representative samples of Masson’s trichrome staining showing increased SAN fibrosis in mice infused with Ang II compared with saline for 3 weeks. Scale bars: 100 μm. (B) Summary data showing increased fibrosis in the SAN (intranodal, P = 0.06, n = 3/group) and in SAN-adjacent tissue (perinodal, *P = 0.03, n = 3/group). (C) Top row: Representative atrial isolate and conduction velocity measurement. Bottom row: Representative pseudocolored isochrones from optical mapping show decreased conduction velocity (CV) in WT mice infused with Ang II for 3 weeks (lower right panel) compared with WT mice infused with saline for 3 weeks (lower left panel). AVJ, atrioventricular junction; AVN, atrioventricular node; IVC, inferior vena cava; LA, left atrium; LAA, left atrial appendage; RAA, right atrial appendage; SVC, superior vena cava. White asterisks denote the earliest activation in the SAN. (D) Summary data for CV measured from Langendorff-perfused hearts isolated from mice infused with Ang II or saline for 3 weeks. CV was measured in the RA (n = 5/group, P = 0.07 baseline and *P = 0.03 Iso) and LA (#P = 0.001 baseline and †P = 0.001 Iso, n = 5/group). The P values represent comparisons between Ang II– and saline-infused mice.
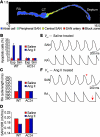
Figure 7. NADPH oxidase mediates Ang II–induced CaMKII oxidation, apoptosis, and SND.
Ang II infusion for 3 weeks in p47–/– mice does not increase ox-CaMKII by immunofluorescence (A) or immunoblotting (B and C). P = 0.54, n = 5/group. The lanes were run on the same gel but were noncontiguous. Scale bars: 50 μm. (D) Unanesthetized and unrestrained p47–/– mice have no significant decrease in resting HR (P = 0.97, n = 15/group) after Ang II compared with saline infusion for 3 weeks. *P < 0.01 between groups. (E) Langendorff-perfused hearts explanted from mice in D show no increase in sinus pauses (n = 5/group). (F) Ang II infusion for 3 weeks in p47–/– mice does not increase CSNRT (P = 0.66 for 100 ms and P = 0.40 for 80 ms; n = 5/group) (G) Summary data showing no increase in TUNEL-positive SAN cells in Ang II– compared with saline-infused p47–/– mice (P = 0.12, n = 5/group). *P = 0.01 between groups.
Comment in
- Oxidized CaMKII: a "heart stopper" for the sinus node?
Huke S, Knollmann BC. Huke S, et al. J Clin Invest. 2011 Aug;121(8):2975-7. doi: 10.1172/JCI58389. Epub 2011 Jul 25. J Clin Invest. 2011. PMID: 21785211 Free PMC article.
Similar articles
- Oxidized CaMKII: a "heart stopper" for the sinus node?
Huke S, Knollmann BC. Huke S, et al. J Clin Invest. 2011 Aug;121(8):2975-7. doi: 10.1172/JCI58389. Epub 2011 Jul 25. J Clin Invest. 2011. PMID: 21785211 Free PMC article. - Protective effects of kaempferol against cardiac sinus node dysfunction via CaMKII deoxidization.
An M, Kim M. An M, et al. Anat Cell Biol. 2015 Dec;48(4):235-43. doi: 10.5115/acb.2015.48.4.235. Epub 2015 Dec 21. Anat Cell Biol. 2015. PMID: 26770873 Free PMC article. - CaMKII in sinoatrial node physiology and dysfunction.
Wu Y, Anderson ME. Wu Y, et al. Front Pharmacol. 2014 Mar 18;5:48. doi: 10.3389/fphar.2014.00048. eCollection 2014. Front Pharmacol. 2014. PMID: 24672485 Free PMC article. Review. - Emerging Signaling Regulation of Sinoatrial Node Dysfunction.
Zheng M, Erhardt S, Cao Y, Wang J. Zheng M, et al. Curr Cardiol Rep. 2023 Jul;25(7):621-630. doi: 10.1007/s11886-023-01885-8. Epub 2023 May 25. Curr Cardiol Rep. 2023. PMID: 37227579 Free PMC article. Review. - Oxidized Ca(2+)/calmodulin-dependent protein kinase II triggers atrial fibrillation.
Purohit A, Rokita AG, Guan X, Chen B, Koval OM, Voigt N, Neef S, Sowa T, Gao Z, Luczak ED, Stefansdottir H, Behunin AC, Li N, El-Accaoui RN, Yang B, Swaminathan PD, Weiss RM, Wehrens XH, Song LS, Dobrev D, Maier LS, Anderson ME. Purohit A, et al. Circulation. 2013 Oct 15;128(16):1748-57. doi: 10.1161/CIRCULATIONAHA.113.003313. Epub 2013 Sep 12. Circulation. 2013. PMID: 24030498 Free PMC article.
Cited by
- Hippo-Yap Signaling Maintains Sinoatrial Node Homeostasis.
Zheng M, Li RG, Song J, Zhao X, Tang L, Erhardt S, Chen W, Nguyen BH, Li X, Li M, Wang J, Evans SM, Christoffels VM, Li N, Wang J. Zheng M, et al. Circulation. 2022 Nov 29;146(22):1694-1711. doi: 10.1161/CIRCULATIONAHA.121.058777. Epub 2022 Nov 1. Circulation. 2022. PMID: 36317529 Free PMC article. - CaMKII oxidation is a critical performance/disease trade-off acquired at the dawn of vertebrate evolution.
Wang Q, Hernández-Ochoa EO, Viswanathan MC, Blum ID, Do DC, Granger JM, Murphy KR, Wei AC, Aja S, Liu N, Antonescu CM, Florea LD, Talbot CC Jr, Mohr D, Wagner KR, Regot S, Lovering RM, Gao P, Bianchet MA, Wu MN, Cammarato A, Schneider MF, Bever GS, Anderson ME. Wang Q, et al. Nat Commun. 2021 May 26;12(1):3175. doi: 10.1038/s41467-021-23549-3. Nat Commun. 2021. PMID: 34039988 Free PMC article. - Natriuretic Peptide Clearance Receptor (NPR-C) Pathway as a Novel Therapeutic Target in Obesity-Related Heart Failure With Preserved Ejection Fraction (HFpEF).
Egom EEA. Egom EEA. Front Physiol. 2021 May 21;12:674254. doi: 10.3389/fphys.2021.674254. eCollection 2021. Front Physiol. 2021. PMID: 34093235 Free PMC article. Review. - Atrial fibrillation and sinus node dysfunction in human ankyrin-B syndrome: a computational analysis.
Wolf RM, Glynn P, Hashemi S, Zarei K, Mitchell CC, Anderson ME, Mohler PJ, Hund TJ. Wolf RM, et al. Am J Physiol Heart Circ Physiol. 2013 May;304(9):H1253-66. doi: 10.1152/ajpheart.00734.2012. Epub 2013 Feb 22. Am J Physiol Heart Circ Physiol. 2013. PMID: 23436330 Free PMC article. - Calmodulin-dependent protein kinase II: linking heart failure and arrhythmias.
Swaminathan PD, Purohit A, Hund TJ, Anderson ME. Swaminathan PD, et al. Circ Res. 2012 Jun 8;110(12):1661-77. doi: 10.1161/CIRCRESAHA.111.243956. Circ Res. 2012. PMID: 22679140 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL 070250/HL/NHLBI NIH HHS/United States
- R01 HL 096652/HL/NHLBI NIH HHS/United States
- R01 HL096652/HL/NHLBI NIH HHS/United States
- R00 HL 096805/HL/NHLBI NIH HHS/United States
- R01 HL 071140/HL/NHLBI NIH HHS/United States
- RR026293/RR/NCRR NIH HHS/United States
- R01 HL070250/HL/NHLBI NIH HHS/United States
- R00 HL096805/HL/NHLBI NIH HHS/United States
- T32 GM007337/GM/NIGMS NIH HHS/United States
- S10 RR026293/RR/NCRR NIH HHS/United States
- R01 HL071140/HL/NHLBI NIH HHS/United States
- T32 GM067795/GM/NIGMS NIH HHS/United States
- R01 HL079031/HL/NHLBI NIH HHS/United States
- R01 HL 079031/HL/NHLBI NIH HHS/United States
- T32 HL007121/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous