The Krüppel-like zinc finger protein GLIS3 transactivates neurogenin 3 for proper fetal pancreatic islet differentiation in mice - PubMed (original) (raw)
The Krüppel-like zinc finger protein GLIS3 transactivates neurogenin 3 for proper fetal pancreatic islet differentiation in mice
Y Yang et al. Diabetologia. 2011 Oct.
Abstract
Aims/hypothesis: Mutations in GLIS3, which encodes a Krüppel-like zinc finger transcription factor, were found to underlie sporadic neonatal diabetes. Inactivation of Glis3 by gene targeting in mice was previously shown to lead to neonatal diabetes, but the underlying mechanism remains largely unknown. We aimed to elucidate the mechanism of action of GLIS family zinc finger 3 (GLIS3) in Glis3 ( -/- ) mice and to further decipher its action in in-vitro systems.
Methods: We created Glis3 ( -/- ) mice and monitored the morphological and biochemical phenotype of their pancreatic islets at different stages of embryonic development. We combined these observations with experiments on Glis3 expressed in cultured cells, as well as in in vitro systems in the presence of other reconstituted components.
Results: In vivo and in vitro analyses placed Glis3 upstream of Neurog3, the endocrine pancreas lineage-defining transcription factor. We found that GLIS3 binds to specific GLIS3-response elements in the Neurog3 promoter, activating Neurog3 gene transcription both directly, and synergistically with hepatic nuclear factor 6 and forkhead box A2.
Conclusions/interpretation: These results indicate that GLIS3 controls fetal islet differentiation via direct transactivation of Neurog3, a perturbation that causes neonatal diabetes in mice.
Figures
Fig. 1
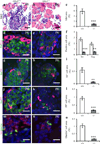
Defective endocrine cell differentiation in Glis3−/− mice at P0. a Histology of pancreas by haematoxylin and eosin (H&E) staining in Glis3+/+ and (b) Glis3−/− mice, with (c) islet area quantification. d, e Immunostaining and (f) quantification of cell areas positive for insulin (Ins, green) and glucagon (Gcg, red). g, h Immunostaining and (i) quantification of cell areas positive for insulin (green) and somatostain (SS, red), for (j–l) insulin (green) and pancreatic polypeptide (PP, red), and for (m–o) insulin (red) and ghrelin (green) in the pancreas of mice as labelled. **p<0.01, ***p<0.001 vs Glis3+/+. Scale bars 20 µm
Fig. 2
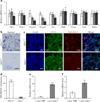
GLIS3 regulates Neurog3 expression in vivo and in vitro. a The mRNA expression of Pdx1, Nkx6-1, Neurog3, Neurod1, Isl1, Hnf6, Foxa2, Hnf1b and Sox9 in total pancreas of E13.5 Glis3+/+ (white bars), Glis3+/− (grey bars) and Glis3−/− (black bars) mice as determined by quantitative RT-PCR (_n_=6). The housekeeping gene cyclophilin Awas used as an internal control. Results are fold change relative to Glis3+/+ mice, shown as the mean±SE; *p<0.05, **p<0.01 vs Glis3+/+ mice. b Neurog3 mRNA expression in the pancreas of Glis3+/+ and Glis3−/− mice at E12.5, assayed by in situ hybridisation. c Immunostaining of PDX1 and NEUROG3 in the pancreas of Glis3+/+ and Glis3−/− mice at E12.5. Nuclei were visualised by DAPI staining. Scale bar (b, c) 20 µm. d Quantification of NEUROG3-positive cells on E12.5 pancreatic immunostaining sections from Glis3+/+ (_n_=5) and Glis3−/− (_n_=10) mice. e mRNA expression of Glis3 and (f) Neurog3 in PDCs transduced with pMSCV-_c-myc_-YFP or -c-myc-Glis3 by quantitative RT-PCR (_n_=4). **p<0.01, ***p<0.001 vs YFP-treated cells
Fig. 3
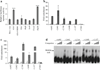
GLIS3 activates Neurog3 expression by directly binding to its promoter. a Promoter reporter assays performed in 832/13 cells co-transfected with the promoter reporter Neurog3 (5.8 kb)-Gluc and Bos-c-myc-Glis3, or -_Glis3_-NDH1, -Pdx1, -Hnf6, -Sox9, -Foxa2 or -Hnf1b (_n_=3). ***p<0.001 vs Bos-c-myc control. b ChIP was performed in PDCs transduced with pMSCV-_c-myc_-YFP (white bars) or -c-myc-Glis3 (black bars), both with anti-c-Myc antibody, and (c) in pooled E13.5 pancreases with anti-GLIS3 antibody (black bars). White bars, IgG. Immunoprecipitated DNA was purified and analysed by quantitative PCR using primers specifically spanning putative GLIS3REs or a control fragment located in intron 1 (+7,963) in Neurog3 promoter. d EMSA using an in vitro translated GLIS3-zinc finger domain peptide was performed with biotin-labelled probes containing putative GLIS3RE sequences as indicated. Five or twenty-five-fold unlabelled wild-type probes were added as competitors. Arrow, specific bands for mobility shift.
Fig. 4
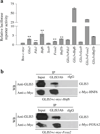
GLIS3 binds to −2,718 GLIS3RE in mouse Neurog3 promoter. a Schematic view of five tandem copies of the wild-type (WT) or mutated (M1–M5) GLIS3RE (−2,718) fused with Neurog3 TATA box to drive expression of luciferase ([GLIS3RE]5-Gluc). Cells (832/13) were co-transfected with (GLIS3RE)5-Gluc and c-myc-Glis3 (grey bars) or Bos-c-myc (white bars) constructs. The activity of each construct was normalised to that obtained with Bos-c-myc. b EMSA with an in vitro translated GLIS3-zinc finger domain (ZFD) peptide was performed using a biotin-labelled probe containing putative −2,718 GLIS3RE sequence as indicated (a). Tenfold non-labelled wild-type or mutated probes were added as competitors. Arrow, specific bands for mobility shift
Fig. 5
GLIS3 transactivates Neurog3 expression synergistically with HNF6 and FOXA2. a HepG2 cells co-transfected with Neurog3 promoter reporter (−5.8 kb to 40 bp)-Gluc and individual c-myc-Glis3, -Pdx1, -Hnf6, -Sox9, -Foxa2 and -Hnf1b or each in combination with c-myc-Glis3. Cells were co-transfected with CMV-β-galactosidase as a control. **p<0.01 vs Bos-c-myc control. b HepG2 cells were co-transfected with Glis3 and c-myc-Hnf6 or -Foxa2. At 48 h after transfection, nuclear proteins were isolated and immunoprecipitated either with a rabbit anti-GLIS3 or rabbit IgG (rIgG) control. The immunoprecipitates were analysed with western blotting (WB) using anti-GLIS3 or anti-c-Myc antibody.
Similar articles
- Glis3 regulates neurogenin 3 expression in pancreatic β-cells and interacts with its activator, Hnf6.
Kim YS, Kang HS, Takeda Y, Hom L, Song HY, Jensen J, Jetten AM. Kim YS, et al. Mol Cells. 2012 Aug;34(2):193-200. doi: 10.1007/s10059-012-0109-z. Epub 2012 Jul 20. Mol Cells. 2012. PMID: 22820919 Free PMC article. - Differential Gene Dosage Effects of Diabetes-Associated Gene GLIS3 in Pancreatic β Cell Differentiation and Function.
Yang Y, Bush SP, Wen X, Cao W, Chan L. Yang Y, et al. Endocrinology. 2017 Jan 1;158(1):9-20. doi: 10.1210/en.2016-1541. Endocrinology. 2017. PMID: 27813676 Free PMC article. - Emerging roles of GLIS3 in neonatal diabetes, type 1 and type 2 diabetes.
Wen X, Yang Y. Wen X, et al. J Mol Endocrinol. 2017 Feb;58(2):R73-R85. doi: 10.1530/JME-16-0232. Epub 2016 Nov 29. J Mol Endocrinol. 2017. PMID: 27899417 Review. - Dissecting Human Gene Functions Regulating Islet Development With Targeted Gene Transduction.
Pauerstein PT, Sugiyama T, Stanley SE, McLean GW, Wang J, Martín MG, Kim SK. Pauerstein PT, et al. Diabetes. 2015 Aug;64(8):3037-49. doi: 10.2337/db15-0042. Epub 2015 Apr 21. Diabetes. 2015. PMID: 25901096 Free PMC article. - GLIS3: A Critical Transcription Factor in Islet β-Cell Generation.
Scoville DW, Jetten AM. Scoville DW, et al. Cells. 2021 Dec 9;10(12):3471. doi: 10.3390/cells10123471. Cells. 2021. PMID: 34943978 Free PMC article. Review.
Cited by
- Fatty acid transport protein-2 regulates glycemic control and diabetic kidney disease progression.
Khan S, Gaivin R, Abramovich C, Boylan M, Calles J, Schelling JR. Khan S, et al. JCI Insight. 2020 Aug 6;5(15):e136845. doi: 10.1172/jci.insight.136845. JCI Insight. 2020. PMID: 32614804 Free PMC article. - The Spatiotemporal Pattern of Glis3 Expression Indicates a Regulatory Function in Bipotent and Endocrine Progenitors during Early Pancreatic Development and in Beta, PP and Ductal Cells.
Kang HS, Takeda Y, Jeon K, Jetten AM. Kang HS, et al. PLoS One. 2016 Jun 7;11(6):e0157138. doi: 10.1371/journal.pone.0157138. eCollection 2016. PLoS One. 2016. PMID: 27270601 Free PMC article. - Neonatal diabetes mutations disrupt a chromatin pioneering function that activates the human insulin gene.
Akerman I, Maestro MA, De Franco E, Grau V, Flanagan S, García-Hurtado J, Mittler G, Ravassard P, Piemonti L, Ellard S, Hattersley AT, Ferrer J. Akerman I, et al. Cell Rep. 2021 Apr 13;35(2):108981. doi: 10.1016/j.celrep.2021.108981. Cell Rep. 2021. PMID: 33852861 Free PMC article. - GLIS3 binds pancreatic beta cell regulatory regions alongside other islet transcription factors.
Scoville DW, Lichti-Kaiser K, Grimm SA, Jetten AM. Scoville DW, et al. J Endocrinol. 2019 Oct;243(1):1-14. doi: 10.1530/JOE-19-0182. J Endocrinol. 2019. PMID: 31340201 Free PMC article. - GLIS1-3: emerging roles in reprogramming, stem and progenitor cell differentiation and maintenance.
Scoville DW, Kang HS, Jetten AM. Scoville DW, et al. Stem Cell Investig. 2017 Sep 27;4:80. doi: 10.21037/sci.2017.09.01. eCollection 2017. Stem Cell Investig. 2017. PMID: 29057252 Free PMC article. Review.
References
- Hamilton-Shield JP. Overview of neonatal diabetes. Endocr Dev. 2007;12:12–23. - PubMed
- Barbetti F. Diagnosis of neonatal and infancy-onset diabetes. Endocr Dev. 2007;11:83–93. - PubMed
- Taha D, Barbar M, Kanaan H, Williamson BJ. Neonatal diabetes mellitus, congenital hypothyroidism, hepatic fibrosis, polycystic kidneys, and congenital glaucoma: a new autosomal recessive syndrome? Am J Med Genet A. 2003;122A:269–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK045641/DK/NIDDK NIH HHS/United States
- R01 DK068037/DK/NIDDK NIH HHS/United States
- P30 DK079638/DK/NIDDK NIH HHS/United States
- P30DK079638/DK/NIDDK NIH HHS/United States
- DK-68037/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases