Chronic neuroinflammation impacts the recruitment of adult-born neurons into behaviorally relevant hippocampal networks - PubMed (original) (raw)
Chronic neuroinflammation impacts the recruitment of adult-born neurons into behaviorally relevant hippocampal networks
Karim Belarbi et al. Brain Behav Immun. 2012 Jan.
Abstract
Growing evidence suggests that adult-born granule cells integrate into hippocampal networks and are required for proper cognitive function. Although neuroinflammation is involved in many disorders associated with cognitive impairment, it remains unknown whether it impacts the recruitment of adult-born neurons into behaviorally relevant hippocampal networks. Under similar behavioral conditions, exploration-induced expression of the immediate-early gene Arc in hippocampal cells has been linked to cellular activity observed by electrophysiological recording. By detecting exploration-induced Arc protein expression, we investigated whether neuroinflammation alters the recruitment of adult-born neurons into behaviorally relevant hippocampal networks. Neuroinflammation was induced in rats by intra-cerebroventricular infusion of lipopolysaccharide for 28 days. Animals received bromodeoxyuridine injections starting on day 29 (5 days) and were euthanized two months later. Persistent lipopolysaccharide-induced neuroinflammation was reliably detected by microglial activation in the hippocampus. Neuroinflammation did not impact the number of adult-born neurons but did alter their migration pattern through the granule cell layer. There was a positive correlation between the density of activated microglia and alterations in the fraction of existing granule neurons expressing Arc, suggesting that neuroinflammation induced a long-term disruption of hippocampal network activity. The proportion of adult-born neurons expressing behaviorally induced Arc was significantly lower in lipopolysaccharide-treated rats than in controls. This observation supports the fact that neuroinflammation significantly impacts adult-born neurons recruitment into hippocampal networks encoding spatial information.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
Fig. 1
Impact of chronic neuroinflammation on neurogenesis and the migration of adult-born neurons two months after LPS infusion terminated. (A) Numbers of activated microglia (OX-6 immunoreactive) within the DG of aCSF- and LPS-treated rats (***p=0.0001 vs. aCSF). (B) The number of BrdU-labeled neurons in the DG enclosed blade did not significantly differ between aCSF-treated and LPS-treated animals (p=0.7577). (C) Index of migration calculated as im = m / w × 100, “m” is the distance between the center of the nucleus and the subgranular zone; “w” is the width of the granule cell layer. (D) The index of migration was higher in LPS-treated rats than in aCSF-treated rats (*p=0.0192).
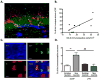
Fig. 2
Impact of chronic neuroinflammation on the expression of behaviorally-induced Arc in existing and adult-born neurons measured two months after LPS infusion terminated. (A) Representative image of double immunofluorescence staining for activated microglia (OX-6 positive) (blue) and Arc protein (red) in the DG of an LPS-treated animal. Nuclei were counterstained with SYTOX Green. (B) There was a significant correlation between the density of activated microglia and the percentage increase of exploration-induced Arc in the DG of LPS-treated rats, above that of aCSF-treated rats (r=0.7063; p=0.0334). (C) Triple immunofluorescence staining for NeuN (Blue), BrdU (green) and Arc (red). The white arrow points to a triple-labeled cell (scale = 10 μm). (D) In aCSF-treated rats, the percentage of BrdU-labeled neurons expressing Arc was higher than the percentage of neurons that expressed Arc in the already existing population of granule cells (**p<0.01). In LPS-treated animals, the percentage of BrdU-labeled neurons expressing Arc was not significantly different than that of the already existing population of neurons. The proportion of BrdU-labeled neurons expressing Arc was significantly lower in LPS-treated animals than in aCSF-treated animals (##p<0.01).
Similar articles
- Integration of new neurons into functional neural networks.
Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Ramirez-Amaya V, et al. J Neurosci. 2006 Nov 22;26(47):12237-41. doi: 10.1523/JNEUROSCI.2195-06.2006. J Neurosci. 2006. PMID: 17122048 Free PMC article. - Neuroinflammation alters the hippocampal pattern of behaviorally induced Arc expression.
Rosi S, Ramirez-Amaya V, Vazdarjanova A, Worley PF, Barnes CA, Wenk GL. Rosi S, et al. J Neurosci. 2005 Jan 19;25(3):723-31. doi: 10.1523/JNEUROSCI.4469-04.2005. J Neurosci. 2005. PMID: 15659610 Free PMC article. - Memantine protects against LPS-induced neuroinflammation, restores behaviorally-induced gene expression and spatial learning in the rat.
Rosi S, Vazdarjanova A, Ramirez-Amaya V, Worley PF, Barnes CA, Wenk GL. Rosi S, et al. Neuroscience. 2006 Nov 3;142(4):1303-15. doi: 10.1016/j.neuroscience.2006.08.017. Epub 2006 Sep 20. Neuroscience. 2006. PMID: 16989956 - Accuracy of hippocampal network activity is disrupted by neuroinflammation: rescue by memantine.
Rosi S, Ramirez-Amaya V, Vazdarjanova A, Esparza EE, Larkin PB, Fike JR, Wenk GL, Barnes CA. Rosi S, et al. Brain. 2009 Sep;132(Pt 9):2464-77. doi: 10.1093/brain/awp148. Epub 2009 Jun 16. Brain. 2009. PMID: 19531533 Free PMC article. - Modulation of adult-born neurons in the inflamed hippocampus.
Belarbi K, Rosi S. Belarbi K, et al. Front Cell Neurosci. 2013 Sep 6;7:145. doi: 10.3389/fncel.2013.00145. Front Cell Neurosci. 2013. PMID: 24046730 Free PMC article. Review.
Cited by
- CCR2 deficiency prevents neuronal dysfunction and cognitive impairments induced by cranial irradiation.
Belarbi K, Jopson T, Arellano C, Fike JR, Rosi S. Belarbi K, et al. Cancer Res. 2013 Feb 1;73(3):1201-10. doi: 10.1158/0008-5472.CAN-12-2989. Epub 2012 Dec 13. Cancer Res. 2013. PMID: 23243025 Free PMC article. - Withania somnifera (L.) Dunal ameliorates neurodegeneration and cognitive impairments associated with systemic inflammation.
Gupta M, Kaur G. Gupta M, et al. BMC Complement Altern Med. 2019 Aug 15;19(1):217. doi: 10.1186/s12906-019-2635-0. BMC Complement Altern Med. 2019. PMID: 31416451 Free PMC article. - Immune Regulation of Adult Neurogenic Niches in Health and Disease.
Chintamen S, Imessadouene F, Kernie SG. Chintamen S, et al. Front Cell Neurosci. 2021 Jan 20;14:571071. doi: 10.3389/fncel.2020.571071. eCollection 2020. Front Cell Neurosci. 2021. PMID: 33551746 Free PMC article. Review. - Adult neurogenesis and mental illness.
Schoenfeld TJ, Cameron HA. Schoenfeld TJ, et al. Neuropsychopharmacology. 2015 Jan;40(1):113-28. doi: 10.1038/npp.2014.230. Epub 2014 Sep 2. Neuropsychopharmacology. 2015. PMID: 25178407 Free PMC article. Review.
References
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. - PubMed
- Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37:26–32. - PubMed
- Barger SW, Basile AS. Activation of microglia by secreted amyloid precursor protein evokes release of glutamate by cystine exchange and attenuates synaptic function. J Neurochem. 2001;76:846–854. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources