Coordinate-based meta-analysis of experimentally induced and chronic persistent neuropathic pain - PubMed (original) (raw)
Review
Coordinate-based meta-analysis of experimentally induced and chronic persistent neuropathic pain
Ulrike Friebel et al. Neuroimage. 2011.
Abstract
Differences in brain activation in experimentally induced and chronic neuropathic pain conditions are useful for understanding central mechanisms leading to chronic neuropathic pain. Many mapping studies investigating both pain conditions are now available, and the latest tools for coordinate-based meta-analysis offer the possibility of random effects statistics. We performed a meta-analysis based on a literature search of published functional magnetic resonance imaging group studies to compare patterns of activity during experimentally induced and chronic neuropathic pain, for the later including four fibromyalgia studies. Stimulus-dependent activation in experimental pain was further divided into "thermal" and "non thermal" stimuli. A conjunction of experimentally induced and chronic neuropathic pain revealed activation of the bilateral secondary somatosensory cortex, right middle cingulate cortex, right inferior parietal lobe, supplementary motor area, right caudal anterior insula, and bilateral thalamus. Primary somatosensory activation was only observed during experimental non-thermal stimulation. Chronic neuropathic pain studies showed increased activation in the left secondary somatosensory cortex, anterior cingulate cortex, and right caudal anterior insula when compared to experimentally induced pain. Activation clusters in the anterior cingulate cortex and caudal anterior insula suggest a strong emotional contribution to the processing of chronic neuropathic pain.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
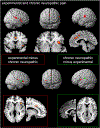
Fig. 1.
Top two image lines: Conjunction analysis of experimental and neuropathic pain. Bottom two image lines: Contrast between conditions. The conjunction as well as the contrast analyses of the conditions “experimentally induced” and “chronic neuropathic pain” projected on the SPM-render brain hemispheres (left and right side view) and slices of the single subject template from SPM. The conjunction analysis “experimental and chronic neuropathic pain” showed activation in bilateral SII, right SMA, bilateral MCC and right ACC, right insula and bilateral thalamus; coronal (y=14), axial (z=−3), sagittal (x=4). The contrast analysis of the conditions “experimental pain" – “chronic neuropathic pain” (red frame) showed activation of the right anterior insula (including right opercular gyrus) and left posterior insula, the right SMA and right MCC; axial (z=7), sagittal (x=12). The contrast analysis of the conditions “chronic neuropathic pain” – “experimental pain” (green frame) showed significant results in left SII, right mid-insula and left ACC; sagittal (x=−2), axial (z=9).
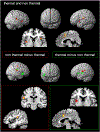
Fig. 2.
The conjunction as well as the contrast analyses of the conditions “thermal” and “non thermal” projected on the SPM-render brain hemispheres (left and right side view) and slices of the single subject template from SPM. The conjunction analysis, “thermal and non-thermal pain” showed significant results in bilateral SII, unilaterally activated SMA and MCC (both in the right hemisphere) and bilateral activation of the insula; axial (z=1), sagittal (x=6). The contrast analysis of the conditions “non thermal” – “thermal” (red frame) showed activation in right SI, bilateral SII, and right mid orbital gyrus; sagittal (x=44), coronal (y=−20). The opposite contrast “thermal” – “non thermal” (green frame) revealed activity in right SII, left middle/posterior insula, left ACC and MCC, right inferior frontal gyrus and bilateral thalamus; sagittal (x=−6), axial (z=7).
Similar articles
- Differential coding of hyperalgesia in the human brain: a functional MRI study.
Maihöfner C, Handwerker HO. Maihöfner C, et al. Neuroimage. 2005 Dec;28(4):996-1006. doi: 10.1016/j.neuroimage.2005.06.049. Epub 2005 Aug 19. Neuroimage. 2005. PMID: 16112876 Clinical Trial. - Functional imaging of brain responses to pain. A review and meta-analysis (2000).
Peyron R, Laurent B, García-Larrea L. Peyron R, et al. Neurophysiol Clin. 2000 Oct;30(5):263-88. doi: 10.1016/s0987-7053(00)00227-6. Neurophysiol Clin. 2000. PMID: 11126640 Review. - Amplified Brain Processing of Dentoalveolar Pressure Stimulus in Persistent Dentoalveolar Pain Disorder Patients.
Moana-Filho EJ, Bereiter DA, Nixdorf DR. Moana-Filho EJ, et al. J Oral Facial Pain Headache. 2015 Fall;29(4):349-62. doi: 10.11607/ofph.1463. J Oral Facial Pain Headache. 2015. PMID: 26485382 Free PMC article. - fMRI evidence of degeneration-induced neuropathic pain in diabetes: enhanced limbic and striatal activations.
Tseng MT, Chiang MC, Chao CC, Tseng WY, Hsieh ST. Tseng MT, et al. Hum Brain Mapp. 2013 Oct;34(10):2733-46. doi: 10.1002/hbm.22105. Epub 2012 Apr 21. Hum Brain Mapp. 2013. PMID: 22522975 Free PMC article. - [Functional brain mapping of pain perception].
Peyron R, Faillenot I. Peyron R, et al. Med Sci (Paris). 2011 Jan;27(1):82-7. doi: 10.1051/medsci/201127182. Med Sci (Paris). 2011. PMID: 21299967 Review. French.
Cited by
- Current Understanding of the Involvement of the Insular Cortex in Neuropathic Pain: A Narrative Review.
Wang N, Zhang YH, Wang JY, Luo F. Wang N, et al. Int J Mol Sci. 2021 Mar 5;22(5):2648. doi: 10.3390/ijms22052648. Int J Mol Sci. 2021. PMID: 33808020 Free PMC article. Review. - Diminished neurokinin-1 receptor availability in patients with two forms of chronic visceral pain.
Jarcho JM, Feier NA, Bert A, Labus JA, Lee M, Stains J, Ebrat B, Groman SM, Tillisch K, Brody AL, London ED, Mandelkern MA, Mayer EA. Jarcho JM, et al. Pain. 2013 Jul;154(7):987-96. doi: 10.1016/j.pain.2013.02.026. Epub 2013 Mar 5. Pain. 2013. PMID: 23582152 Free PMC article. Clinical Trial. - Aberrant cerebral blood flow and functional connectivity in patients with vestibular migraine: a resting-state ASL and fMRI study.
Chen Z, Liu Y, Lin C, Li Z, Shan J, Duan Z, Rong L, Wei X, Xiao L, Liu H. Chen Z, et al. J Headache Pain. 2024 May 21;25(1):84. doi: 10.1186/s10194-024-01792-5. J Headache Pain. 2024. PMID: 38773396 Free PMC article. - Insula-cingulate structural and functional connectivity: an ultra-high field MRI study.
Cormie MA, Kaya B, Hadjis GE, Mouseli P, Moayedi M. Cormie MA, et al. Cereb Cortex. 2023 Aug 23;33(17):9787-9801. doi: 10.1093/cercor/bhad244. Cereb Cortex. 2023. PMID: 37429832 Free PMC article. - Sex differences in brain response to anticipated and experienced visceral pain in healthy subjects.
Kano M, Farmer AD, Aziz Q, Giampietro VP, Brammer MJ, Williams SC, Fukudo S, Coen SJ. Kano M, et al. Am J Physiol Gastrointest Liver Physiol. 2013 Apr 15;304(8):G687-99. doi: 10.1152/ajpgi.00385.2012. Epub 2013 Feb 7. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23392235 Free PMC article.
References
- Albuquerque RJ, de Leeuw R, Carlson CR, Okeson JP, Miller CS, Andersen AH, 2006. Cerebral activation during thermal stimulation of patients who have burning mouth disorder: an fMRI study. Pain 122, 223–234. - PubMed
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK, 2005. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain 9, 463–484. - PubMed
- Baron R, 2006. Mechanisms of disease: neuropathic pain—a clinical perspective. Nat. Clin. Pract. Neurol. 2, 95–106. - PubMed
- Becerra LR, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comite AR, Gonzalez GR, Borsook D, 1999. Human brain activation under controlled thermal stimulation and habituation to noxious heat: an fMRI study. Magn. Reson. Med. 41, 1044–1057. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources