A standardized protocol for repeated social defeat stress in mice - PubMed (original) (raw)
A standardized protocol for repeated social defeat stress in mice
Sam A Golden et al. Nat Protoc. 2011.
Erratum in
- Corrigendum: a standardized protocol for repeated social defeat stress in mice.
Golden SA, Covington HE 3rd, Berton O, Russo SJ. Golden SA, et al. Nat Protoc. 2015 Apr;10(4):643. doi: 10.1038/nprot0415-644a. Epub 2015 Mar 26. Nat Protoc. 2015. PMID: 25811900 No abstract available.
Abstract
A major impediment to novel drug development has been the paucity of animal models that accurately reflect symptoms of affective disorders. In animal models, prolonged social stress has proven to be useful in understanding the molecular mechanisms underlying affective-like disorders. When considering experimental approaches for studying depression, social defeat stress, in particular, has been shown to have excellent etiological, predictive, discriminative and face validity. Described here is a protocol whereby C57BL/6J mice that are repeatedly subjected to bouts of social defeat by a larger and aggressive CD-1 mouse results in the development of a clear depressive-like syndrome, characterized by enduring deficits in social interactions. Specifically, the protocol consists of three important stages, beginning with the selection of aggressive CD-1 mice, followed by agonistic social confrontations between the CD-1 and C57BL/6J mice, and concluding with the confirmation of social avoidance in subordinate C57BL/6J mice. The automated detection of social avoidance allows a marked increase in throughput, reproducibility and quantitative analysis. This protocol is highly adaptable, but in its most common form it requires 3-4 weeks for completion.
Figures
Figure 1
Picture of the standard hamster cage used in repeated social defeat stress experiments. The resident aggressor is permanently housed on one side of the perforated divider, and all defeats are performed within that compartment while rotating C57BL/6J intruders across defeat days so that these experimental animals do not habituate to a single aggressor. Note that care must be taken to ensure that the divider is firmly secure, thereby preventing mice from escaping their overnight compartments. Social defeat experiments must follow all governmental and institutional guidelines for care and use of laboratory animals.
Figure 2
Schematic of the social interaction arena and representative front and side pictures of a wire-mesh enclosure. (a) Top-down view of the social interaction arena, delineated with zones and dimensions. (b) Picture of wire-mesh enclosure used to secure the target CD-1 during the `target present' portion of the social interaction test. Note that this is custom designed, and, assuming that dimensions are held constant, it can be fabricated with some flexibility in construction materials. Of importance is the ability for visual and olfactory cue transmission, with physical separation of the target aggressor and defeated mouse. On the left is a front view, and on the right a side view, of the same wire-mesh enclosure.
Figure 3
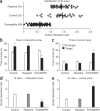
Repeated social defeat stress induces avoidance behavior in susceptible mice. (a) Repeated social defeat stress results in a spectrum of avoidance behavior, divided between susceptible and resilient phenotypes as a function of their social interaction (SI) ratio score. This is the ratio of time a mouse spends in the interaction zone in the presence of a target CD-1 compared with the absence of a target CD-1. (b,c) Susceptible mice spend significantly more time in the corner zone than in the interaction zone, whereas resilient mice spend comparable amounts of time in the interaction zone to control mice that have never undergone a defeat procedure. Both control and resilient mice spend significantly more time in the interaction zone when a target is present. (d,e) Social avoidance behavior can also be expressed as a social interaction ratio. In this panel, the same data are shown in both manners, for comparison. Error bars represent means ± s.e.m. *P < 0.05, multivariate ANOVA/ANOVA. All data shown were collected while conforming to governmental and institutional guidelines for care and use of laboratory animals.
Similar articles
- Influence of aging on the behavioral phenotypes of C57BL/6J mice after social defeat.
Oizumi H, Kuriyama N, Imamura S, Tabuchi M, Omiya Y, Mizoguchi K, Kobayashi H. Oizumi H, et al. PLoS One. 2019 Sep 3;14(9):e0222076. doi: 10.1371/journal.pone.0222076. eCollection 2019. PLoS One. 2019. PMID: 31479487 Free PMC article. - Reproducible induction of depressive-like behavior in C57BL/6J mice exposed to chronic social defeat stress with a modified sensory contact protocol.
Kamimura Y, Kuwagaki E, Hamano S, Kobayashi M, Yamada Y, Takahata Y, Yoshimoto W, Morimoto H, Yasukawa T, Uozumi Y, Nagasawa K. Kamimura Y, et al. Life Sci. 2021 Oct 1;282:119821. doi: 10.1016/j.lfs.2021.119821. Epub 2021 Jul 13. Life Sci. 2021. PMID: 34271059 - Testing Depression in Mice: a Chronic Social Defeat Stress Model.
Kim HD, Call T, Carotenuto S, Johnson R, Ferguson D. Kim HD, et al. Bio Protoc. 2017 Apr 5;7(7):e2203. doi: 10.21769/BioProtoc.2203. eCollection 2017 Apr 5. Bio Protoc. 2017. PMID: 34541213 Free PMC article. - Defeat stress in rodents: From behavior to molecules.
Hammels C, Pishva E, De Vry J, van den Hove DL, Prickaerts J, van Winkel R, Selten JP, Lesch KP, Daskalakis NP, Steinbusch HW, van Os J, Kenis G, Rutten BP. Hammels C, et al. Neurosci Biobehav Rev. 2015 Dec;59:111-40. doi: 10.1016/j.neubiorev.2015.10.006. Epub 2015 Oct 22. Neurosci Biobehav Rev. 2015. PMID: 26475995 Review. - Aggression and defeat: persistent effects on cocaine self-administration and gene expression in peptidergic and aminergic mesocorticolimbic circuits.
Miczek KA, Covington HE 3rd, Nikulina EM Jr, Hammer RP. Miczek KA, et al. Neurosci Biobehav Rev. 2004 Jan;27(8):787-802. doi: 10.1016/j.neubiorev.2003.11.005. Neurosci Biobehav Rev. 2004. PMID: 15019428 Review.
Cited by
- Social stress in adolescents induces depression and brain-region-specific modulation of the transcription factor MAX.
Resende LS, Amaral CE, Soares RB, Alves AS, Alves-Dos-Santos L, Britto LR, Chiavegatto S. Resende LS, et al. Transl Psychiatry. 2016 Oct 11;6(10):e914. doi: 10.1038/tp.2016.202. Transl Psychiatry. 2016. PMID: 27727240 Free PMC article. - Rationale, Relevance, and Limits of Stress-Induced Psychopathology in Rodents as Models for Psychiatry Research: An Introductory Overview.
Italia M, Forastieri C, Longaretti A, Battaglioli E, Rusconi F. Italia M, et al. Int J Mol Sci. 2020 Oct 9;21(20):7455. doi: 10.3390/ijms21207455. Int J Mol Sci. 2020. PMID: 33050350 Free PMC article. Review. - Targeting cAMP in D1-MSNs in the nucleus accumbens, a new rapid antidepressant strategy.
Zhang Y, Gao J, Li N, Xu P, Qu S, Cheng J, Wang M, Li X, Song Y, Xiao F, Yang X, Liu J, Hong H, Mu R, Li X, Wang Y, Xu H, Xie Y, Gao T, Wang G, Aa J. Zhang Y, et al. Acta Pharm Sin B. 2024 Feb;14(2):667-681. doi: 10.1016/j.apsb.2023.12.005. Epub 2023 Dec 19. Acta Pharm Sin B. 2024. PMID: 38322327 Free PMC article. - Striatal Shati/Nat8l-BDNF pathways determine the sensitivity to social defeat stress in mice through epigenetic regulation.
Miyanishi H, Muramatsu SI, Nitta A. Miyanishi H, et al. Neuropsychopharmacology. 2021 Aug;46(9):1594-1605. doi: 10.1038/s41386-021-01033-2. Epub 2021 Jun 7. Neuropsychopharmacology. 2021. PMID: 34099867 Free PMC article. - Region- and receptor-specific effects of chronic social stress on the central serotonergic system in mice.
Carneiro-Nascimento S, Powell W, Uebel M, Buerge M, Sigrist H, Patterson M, Pryce CR, Opacka-Juffry J. Carneiro-Nascimento S, et al. IBRO Neurosci Rep. 2021 Feb 3;10:8-16. doi: 10.1016/j.ibneur.2020.11.001. eCollection 2021 Jun. IBRO Neurosci Rep. 2021. PMID: 33861815 Free PMC article.
References
- Greenberg PE, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000? J. Clin. Psychiatry. 2003;64:1465–1475. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 MH087581/MH/NIMH NIH HHS/United States
- R01 MH090264/MH/NIMH NIH HHS/United States
- R01 MH090264-01A1/MH/NIMH NIH HHS/United States
- 1R01MH090264-01A1/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical