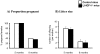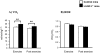Liver-derived IGF-I regulates mean life span in mice - PubMed (original) (raw)
Liver-derived IGF-I regulates mean life span in mice
Johan Svensson et al. PLoS One. 2011.
Abstract
Background: Transgenic mice with low levels of global insulin-like growth factor-I (IGF-I) throughout their life span, including pre- and postnatal development, have increased longevity. This study investigated whether specific deficiency of liver-derived, endocrine IGF-I is of importance for life span.
Methods and findings: Serum IGF-I was reduced by approximately 80% in mice with adult, liver-specific IGF-I inactivation (LI-IGF-I(-/-) mice), and body weight decreased due to reduced body fat. The mean life span of LI-IGF-I(-/-) mice (n = 84) increased 10% vs. control mice (n = 137) (Cox's test, p<0.01), mainly due to increased life span (16%) of female mice [LI-IGF-I(-/-) mice (n = 31): 26.7±1.1 vs. control (n = 67): 23.0±0.7 months, p<0.001]. Male LI-IGF-I(-/-) mice showed only a tendency for increased longevity (p = 0.10). Energy expenditure, measured as oxygen consumption during and after submaximal exercise, was increased in the LI-IGF-I(-/-) mice. Moreover, microarray and RT-PCR analyses showed consistent regulation of three genes (heat shock protein 1A and 1B and connective tissue growth factor) in several body organs in the LI-IGF-I(-/-) mice.
Conclusions: Adult inactivation of liver-derived, endocrine IGF-I resulted in moderately increased mean life span. Body weight and body fat decreased in LI-IGF-I(-/-) mice, possibly due to increased energy expenditure during exercise. Genes earlier reported to modulate stress response and collagen aging showed consistent regulation, providing mechanisms that could underlie the increased mean life span in the LI-IGF-I(-/-) mice.
Conflict of interest statement
Competing Interests: Olle Isaksson was co-founder of Tercica Inc. that produced IGF-I for use in children of short stature. Tercica Inc. was acquired by Ipsen June 2008. Olle Isaksson has no current ownership of stocks or options in Tercica or Ipsen. None of the other authors has any conflict of interest. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Figures
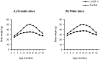
Figure 1. Reduced body weight in LI-IGF-I-/- mice.
Body weight in A) female and B) male mice. Body weight was determined every third month during 3-30 months of age. Between 3 and 27 months of age, body weight was significantly lower in LI-IGF-I-/- compared with control mice in the total number of mice and also in the male and female subgroups (all analyses p<0.001). The vertical bars indicate the SE for the mean values shown.
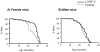
Figure 2. Increased mean life span of female LI-IGF-I-/- mice.
A) Female LI-IGF-I-/- mice live 16% longer than female control mice (26.7 ± 1.1 vs. 23.0 ± 0.7 months, p<0.01 using unpaired _t_-test; Kaplan-Meyer analysis p<0.001, Cox's test). B) Although not statistically significant, male LI-IGF-I-/- mice live 6% longer than male control mice (23.5 ± 0.8 vs. 22.1 ± 0.7 months, p = 0.17 using unpaired t-test; Kaplan-Meyer analysis: p = 0.10, Cox's test).
Figure 3. Normal fertility in female LI-IGF-I-/- mice.
A) The percentage of pregnant female mice in relation to the total number of female mice in each group. B) Litter size when female LI-IGF-I-/- mice were mated with age-matched male control mice at 5 and 8 months of age (n = 12-14 in each group). The experiments showed normal fertility in female LI-IGF-I-/- mice. In Figure 3B, the error bars indicate the standard error for the mean values shown.
Figure 4. Increased oxygen consumption during submaximal exercise and post-exercise in LI-IGF-I-/- mice.
A) The volume of O2 consumed (VO2) and B) respiratory exchange ratio (RER) during submaximal exercise (30 min) and post-exercise (20 min) in 18-month-old female control (n = 7) and LI-IGF-I-/- (n = 7) mice. The vertical bars indicate the SE for the mean values shown. ** p<0.01 vs. control mice
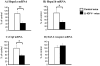
Figure 5. Decreased mRNA levels of Hspa1a, Hspa1b, and Ctgf in LI-IGF-I-/- mice.
To confirm the microarray findings, RT-PCR analyses were performed on skeletal muscle (triceps) samples from individual mice. The RT-PCR analyses showed reduced mRNA levels of A) heat shock protein 1A (Hspa1a), B) heat shock protein 1B (Hspa1b), and C) connective tissue growth factor (Ctgf). D) IGF-I receptor mRNA level was unchanged. The mRNA levels in LI-IGF-I-/- mice (n = 7) are expressed as percent of that in control mice (n = 6). The vertical bars indicate the SE for the mean values shown.* p<0.05 ** p<0.01 vs. control mice.
Similar articles
- Liver-derived IGF-I regulates kidney size, sodium reabsorption, and renal IGF-II expression.
Svensson J, Tivesten A, Sjögren K, Isaksson O, Bergström G, Mohan S, Mölne J, Isgaard J, Ohlsson C. Svensson J, et al. J Endocrinol. 2007 Jun;193(3):359-66. doi: 10.1677/JOE-07-0024. J Endocrinol. 2007. PMID: 17535874 - Congenic mice with low serum IGF-I have increased body fat, reduced bone mineral density, and an altered osteoblast differentiation program.
Rosen CJ, Ackert-Bicknell CL, Adamo ML, Shultz KL, Rubin J, Donahue LR, Horton LG, Delahunty KM, Beamer WG, Sipos J, Clemmons D, Nelson T, Bouxsein ML, Horowitz M. Rosen CJ, et al. Bone. 2004 Nov;35(5):1046-58. doi: 10.1016/j.bone.2004.07.008. Bone. 2004. PMID: 15542029 - A model for tissue-specific inducible insulin-like growth factor-I (IGF-I) inactivation to determine the physiological role of liver-derived IGF-I.
Sjögren K, Jansson JO, Isaksson OG, Ohlsson C. Sjögren K, et al. Endocrine. 2002 Dec;19(3):249-56. doi: 10.1385/ENDO:19:3:249. Endocrine. 2002. PMID: 12624424 Review. - Effects of liver-derived insulin-like growth factor I on bone metabolism in mice.
Sjögren K, Sheng M, Movérare S, Liu JL, Wallenius K, Törnell J, Isaksson O, Jansson JO, Mohan S, Ohlsson C. Sjögren K, et al. J Bone Miner Res. 2002 Nov;17(11):1977-87. doi: 10.1359/jbmr.2002.17.11.1977. J Bone Miner Res. 2002. PMID: 12412805 - A transgenic model to determine the physiological role of liver-derived insulin-like growth factor I.
Sjögren K, Jansson JO, Isaksson OG, Ohlsson C. Sjögren K, et al. Minerva Endocrinol. 2002 Dec;27(4):299-311. Minerva Endocrinol. 2002. PMID: 12511852 Review.
Cited by
- Mex3c mutation reduces adiposity and increases energy expenditure.
Jiao Y, George SK, Zhao Q, Hulver MW, Hutson SM, Bishop CE, Lu B. Jiao Y, et al. Mol Cell Biol. 2012 Nov;32(21):4350-62. doi: 10.1128/MCB.00452-12. Epub 2012 Aug 27. Mol Cell Biol. 2012. PMID: 22927639 Free PMC article. - An experimental model of partial insulin-like growth factor-1 deficiency in mice.
Castilla-Cortazar I, Guerra L, Puche JE, Muñoz U, Barhoum R, Escudero E, Lavandera JL. Castilla-Cortazar I, et al. J Physiol Biochem. 2014 Mar;70(1):129-39. doi: 10.1007/s13105-013-0287-y. Epub 2013 Sep 18. J Physiol Biochem. 2014. PMID: 24043429 - Hyperinsulinemia in Obesity, Inflammation, and Cancer.
Zhang AMY, Wellberg EA, Kopp JL, Johnson JD. Zhang AMY, et al. Diabetes Metab J. 2021 May;45(3):285-311. doi: 10.4093/dmj.2020.0250. Epub 2021 Mar 29. Diabetes Metab J. 2021. PMID: 33775061 Free PMC article. Review. - ROLE of IGF-1 System in the Modulation of Longevity: Controversies and New Insights From a Centenarians' Perspective.
Vitale G, Pellegrino G, Vollery M, Hofland LJ. Vitale G, et al. Front Endocrinol (Lausanne). 2019 Feb 1;10:27. doi: 10.3389/fendo.2019.00027. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 30774624 Free PMC article. Review. - Growth signaling and longevity in mouse models.
Kim SS, Lee CK. Kim SS, et al. BMB Rep. 2019 Jan;52(1):70-85. doi: 10.5483/BMBRep.2019.52.1.299. BMB Rep. 2019. PMID: 30545442 Free PMC article. Review.
References
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. - PubMed
- Kimura K, Tissenbaum H, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. - PubMed
- Clancy D, Gems D, Harshman L, Oldham S, Stocker H, et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. - PubMed
- Tatar M, Kopelman A, Epstein D, Tu M, Yin C, et al. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. - PubMed
- Brown-Borg H, Borg K, Meliska C, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases