A virus-induced gene silencing approach to understanding alkaloid metabolism in Catharanthus roseus - PubMed (original) (raw)
A virus-induced gene silencing approach to understanding alkaloid metabolism in Catharanthus roseus
David K Liscombe et al. Phytochemistry. 2011 Nov.
Abstract
The anticancer agents vinblastine and vincristine are bisindole alkaloids derived from coupling vindoline and catharanthine, monoterpenoid indole alkaloids produced exclusively by the Madagascar periwinkle (Catharanthus roseus). Industrial production of vinblastine and vincristine currently relies on isolation from C. roseus leaves, a process that affords these compounds in 0.0003-0.01% yields. Metabolic engineering efforts to either improve alkaloid content or provide alternative sources of the bisindole alkaloids ultimately rely on the isolation and characterization of the genes involved. Several vindoline biosynthetic genes have been isolated, and the cellular and subcellular organization of the corresponding enzymes has been well studied. However, due to the leaf-specific localization of vindoline biosynthesis, and the lack of production of this precursor in cell suspension and hairy root cultures of C. roseus, further elucidation of this pathway demands the development of reverse genetics approaches to assay gene function in planta. The bipartite pTRV vector system is a Tobacco Rattle Virus-based virus-induced gene silencing (VIGS) platform that has provided efficient and effective means to assay gene function in diverse plant systems. A VIGS method was developed herein to investigate gene function in C. roseus plants using the pTRV vector system. The utility of this approach in understanding gene function in C. roseus leaves is demonstrated by silencing known vindoline biosynthetic genes previously characterized in vitro.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
Figure 1
Proposed biosynthesis of vindoline from tabersonine in C. roseus, and incorporation of vindoline into the bisindole alkaloids, vinblastine and vincristine. Enzyme names are indicated in bold above solid black arrows. Cognate cDNAs have been isolated for all steps in the conversion of tabersonine to vindoline, except for the so-called hydration step (dotted arrow). Grey arrows indicate multiple enzymatic steps. Abbreviations: T16H, tabersonine 16-hydroxylase; 16OMT, 16-hydroxytabersonine 16-_O_-methyltransferase; NMT, 16-methoxy-2,3-dihydro-3-hydroxytabersonine _N_-methyltransferase; D4H, desacetoxyvindoline 4-hydroxylase; DAT, deacetylvindoline 4-_O_-acetyltransferase; PRX1, anhydrovinblastine synthase.
Figure 2
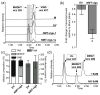
Silencing of protoporphyrin IX magnesium chelatase gene (ChlH) as a visual marker for virus-induced gene silencing. (a) Representative C. roseus plant 2 weeks after infiltration with pTRV1/pTRV2-ChlH(F3R1) (1:1; _ChlH_-vigs) exhibiting photobleached phenotype. (b) Representative C. roseus plant 2 weeks after infiltration with pTRV1/empty pTRV2 (1:1; EV). (c) Fold change in ChlH and vindoline (VIND) biosynthetic gene transcripts levels in leaves of EV and _ChlH_-vigs plants relative to mock-infiltrated (M) plants, as determined by quantitative real-time PCR. Data represent mean ± SE of at least three technical replicates performed with cDNA prepared from 8 (EV) and 3 (_ChlH_-vigs) individual plants. Asterisks indicate significant differences between EV and mock, or EV and _ChlH-vigs plants using Student’s t-test (*, P < 0.05; **, _P_ < 0.0001). **(d)** Alkaloid composition and total alkaloids in leaves of M, EV, and _ChlH_-vigs plants. Data represent mean ± SE alkaloid composition (as % of total) or total alkaloid content (normalized to M) from 5 (M), 8 (EV) and 3 (_ChlH_-vigs) individual plants. Asterisks indicate significant differences between EV and M, or _ChlH-_vigs and M plants using Student’s t-test (*, _P_ < 0.05). Total leaf alkaloid content of from _ChlH_-vigs plants compared to EV plants was not significantly different (_P > 0.05). Abbreviated alkaloid and biosynthetic gene (enzyme) names are defined in Figure 1.
Figure 3
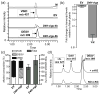
Virus-induced silencing of 16-methoxy-2,3-dihydro-3-hydroxytabersonine _N_-methyltransferase (NMT) transcripts in C. roseus. (a) Selected ion chromatograms (m/z 385+457) showing relative vindoline (VIND, m/z 457) and 16-methoxy-2,3-dihydro-3-hydroxytabersonine (MHDHT, m/z 385) in mock infected (M), empty vector infected (EV), and least (_NMT_-vigs-1) and most (_NMT_-vigs-1a) extreme phenotypes observed for pTRV2-NMT infected plants. (b) Relative expression of NMT in EV and _NMT_-vigs plants compared to M plants, measured by quantitative real-time PCR. Data represent mean ± SE of at least three technical replicates performed with cDNA prepared from 8 (EV) and 7 (_NMT_-vigs) individual plants. Asterisk indicates significant difference between _NMT_-vigs and EV plants using Student’s t-test (P < 0.05). (c) Mean alkaloid composition and total alkaloid content (relative to M) in EV and _NMT_-vigs plants. Data represent mean ± SE alkaloid composition (as % of total) or total alkaloid content (normalized to M) from 8 (EV) and 7 (_NMT_-vigs) individual plants. (d) Selected ion chromatogram (m/z 327+385+399) of in vitro rescue assay demonstrating the _S_-adenosyl-L-methionine (SAM)-dependent conversion of MHDHT (m/z 385) accumulating in _NMT_-vigs plants to desacetoxyvindoline (DESV, m/z 399) by recombinant NMT. Abbreviated alkaloid names are defined in Fig. 1; I.S., ajmaline internal standard (m/z 327).
Figure 4
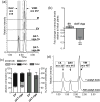
Virus-induced silencing of desacetoxyvindoline 4-hydroxylase (D4H) transcripts in C. roseus. (a) Selected ion chromatograms (m/z 399 and m/z 457) showing relative vindoline (VIND, m/z 457, grey trace) and desacetoxyvindoline (DESV m/z 399, black trace) in mock infected (M), empty vector infected (EV), and least (_D4H_-vigs-4b) and most (_D4H_-vigs-6b) extreme phenotypes observed for pTRV2-D4H infected plants. (b) Relative expression of D4H in EV and _D4H_-vigs plants compared to M plants, measured by quantitative real-time PCR. Data represent mean ± SE of at least three technical replicates performed with cDNA prepared from 8 (EV) and 7 (_D4H_-vigs) individual plants. Asterisks indicate significant difference between _D4H_-vigs and EV plants using Student’s t-test (P < 0.0001). (c) Mean alkaloid composition and total alkaloid content (relative to M) in EV and _D4H_-vigs plants. Data represent mean ± SE alkaloid composition (as % of total) or total alkaloid content (normalized to M) from 8 (EV) and 7 (_D4H_-vigs) individual plants. (d) Selected ion chromatogram (m/z 327+399+415) of in vitro D4H rescue assay demonstrating the α-ketoglutarate (α-KG)-dependent conversion of DESV (m/z 399) accumulating in _D4H_-vigs plants to deacetylvindoline (DAV, m/z 415) using crude, soluble protein extract from young leaves of wild type C. roseus plants. Abbreviated alkaloid names are defined in Fig. 1; I.S., ajmaline internal standard (m/z 327).
Figure 5
Virus-induced silencing of deacetylvindoline 4-_O_-acetyltransferase (DAT) transcripts in C. roseus. (a) Selected ion chromatograms (m/z 415+457) showing relative vindoline (VIND, m/z 457) and deacetylvindoline (DAV, m/z 415) in mock infected (M), empty vector infected (EV), and least (_DAT_-vigs-1b) and most (_DAT_-vigs-6b) extreme phenotypes observed for _DAT_-silenced plants. (b) Relative expression of DAT in EV and _DAT_-vigs plants compared to M plants, measured by quantitative real-time PCR. Data represent mean ± SE of at least three technical replicates performed with cDNA prepared from 8 (EV) and 7 (_DAT_-vigs) individual plants. Asterisks indicate significant difference between _DAT_-vigs and EV plants using Student’s t-test (P < 0.001). (c) Mean alkaloid composition and total alkaloid content (relative to M) in empty vector and _DAT_-vigs plants. Data represent mean ± SE alkaloid composition (as % of total) or total alkaloid content (normalized to M) from 8 (EV) and 7 (_DAT_-vigs) individual plants. (d) Selected ion chromatogram (m/z 327+415+457) of in vitro DAT rescue assays demonstrating the acetyl-CoA-dependent conversion of DAV (m/z 415) accumulating in _DAT_-vigs plants to VIND (m/z 415) using crude, soluble protein extract from methyl jasmonate-elicited C. roseus seedlings. Abbreviated alkaloid names are defined in Fig. 1; I.S., ajmaline internal standard (m/z 327).
Similar articles
- Completion of the seven-step pathway from tabersonine to the anticancer drug precursor vindoline and its assembly in yeast.
Qu Y, Easson ML, Froese J, Simionescu R, Hudlicky T, De Luca V. Qu Y, et al. Proc Natl Acad Sci U S A. 2015 May 12;112(19):6224-9. doi: 10.1073/pnas.1501821112. Epub 2015 Apr 27. Proc Natl Acad Sci U S A. 2015. PMID: 25918424 Free PMC article. - Virus-induced gene silencing in Catharanthus roseus by biolistic inoculation of tobacco rattle virus vectors.
Carqueijeiro I, Masini E, Foureau E, Sepúlveda LJ, Marais E, Lanoue A, Besseau S, Papon N, Clastre M, Dugé de Bernonville T, Glévarec G, Atehortùa L, Oudin A, Courdavault V. Carqueijeiro I, et al. Plant Biol (Stuttg). 2015 Nov;17(6):1242-6. doi: 10.1111/plb.12380. Epub 2015 Aug 30. Plant Biol (Stuttg). 2015. PMID: 26284695 - Enzymology of indole alkaloid biosynthesis in Catharanthus roseus.
Misra N, Luthra R, Kumar S. Misra N, et al. Indian J Biochem Biophys. 1996 Aug;33(4):261-73. Indian J Biochem Biophys. 1996. PMID: 8936815 Review. - Biotechnological advancements in Catharanthus roseus (L.) G. Don.
Das A, Sarkar S, Bhattacharyya S, Gantait S. Das A, et al. Appl Microbiol Biotechnol. 2020 Jun;104(11):4811-4835. doi: 10.1007/s00253-020-10592-1. Epub 2020 Apr 17. Appl Microbiol Biotechnol. 2020. PMID: 32303816 Review.
Cited by
- Improved virus-induced gene silencing allows discovery of a serpentine synthase gene in Catharanthus roseus.
Yamamoto K, Grzech D, Koudounas K, Stander EA, Caputi L, Mimura T, Courdavault V, O'Connor SE. Yamamoto K, et al. Plant Physiol. 2021 Oct 5;187(2):846-857. doi: 10.1093/plphys/kiab285. Plant Physiol. 2021. PMID: 34608956 Free PMC article. - Biosynthesis and regulation of terpenoid indole alkaloids in Catharanthus roseus.
Zhu J, Wang M, Wen W, Yu R. Zhu J, et al. Pharmacogn Rev. 2015 Jan-Jun;9(17):24-8. doi: 10.4103/0973-7847.156323. Pharmacogn Rev. 2015. PMID: 26009689 Free PMC article. Review. - Two Tabersonine 6,7-Epoxidases Initiate Lochnericine-Derived Alkaloid Biosynthesis in Catharanthus roseus.
Carqueijeiro I, Brown S, Chung K, Dang TT, Walia M, Besseau S, Dugé de Bernonville T, Oudin A, Lanoue A, Billet K, Munsch T, Koudounas K, Melin C, Godon C, Razafimandimby B, de Craene JO, Glévarec G, Marc J, Giglioli-Guivarc'h N, Clastre M, St-Pierre B, Papon N, Andrade RB, O'Connor SE, Courdavault V. Carqueijeiro I, et al. Plant Physiol. 2018 Aug;177(4):1473-1486. doi: 10.1104/pp.18.00549. Epub 2018 Jun 22. Plant Physiol. 2018. PMID: 29934299 Free PMC article. - The role of the Golden2-like (GLK) transcription factor in regulating terpenoid indole alkaloid biosynthesis in Catharanthus roseus.
Cole-Osborn LF, McCallan SA, Prifti O, Abu R, Sjoelund V, Lee-Parsons CWT. Cole-Osborn LF, et al. Plant Cell Rep. 2024 May 14;43(6):141. doi: 10.1007/s00299-024-03208-9. Plant Cell Rep. 2024. PMID: 38743349 Free PMC article. - Artificial microRNA mediated gene silencing in plants: progress and perspectives.
Tiwari M, Sharma D, Trivedi PK. Tiwari M, et al. Plant Mol Biol. 2014 Sep;86(1-2):1-18. doi: 10.1007/s11103-014-0224-7. Epub 2014 Jul 15. Plant Mol Biol. 2014. PMID: 25022825 Review.
References
- Aerts RJ, Gisi D, de Carolis E, De Luca V, Baumann TW. Methyl jasmonate vapor increases the developmentally controlled synthesis of alkaloids in Catharanthus and Cinchona seedlings. Plant J. 1994;5:635–643.
- Burch-Smith TM, Anderson JC, Martin GB, Dinesh-Kumar SP. Applications and advantages of virus-induced gene silencing for gene function studies in plants. Plant J. 2004;39:734–746. - PubMed
- Courdavault V, Thiersault M, Courtois M, Gantet P, Oudin A, Doireau P, St-Pierre B, Giglioli-Guivarc’h N. CaaX-prenyltransferases are essential for expression of genes involvedin the early stages of monoterpenoid biosynthetic pathway in Catharanthus roseus cells. Plant Mol Biol. 2005;57:855–870. - PubMed
- De Carolis E, De Luca V. Purification, characterization, and kinetic analysis of a 2-oxoglutarate-dependent dioxygenase involved in vindoline biosynthesis from Catharanthus roseus. J Biol Chem. 1993;268:5504–5511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM074820-01A2/GM/NIGMS NIH HHS/United States
- R01 GM074820-02/GM/NIGMS NIH HHS/United States
- R01 GM074820-03/GM/NIGMS NIH HHS/United States
- R01 GM074820-05/GM/NIGMS NIH HHS/United States
- R01 GM074820-06/GM/NIGMS NIH HHS/United States
- R01 GM074820-04/GM/NIGMS NIH HHS/United States
- BBS/E/J/000C0654/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- R01 GM074820/GM/NIGMS NIH HHS/United States
- GM074820/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources