Boundaries, junctions and transitions in the gastrointestinal tract - PubMed (original) (raw)
Review
Boundaries, junctions and transitions in the gastrointestinal tract
Adrianna K San Roman et al. Exp Cell Res. 2011.
Abstract
Contiguous regions along the mammalian gastrointestinal tract, from the esophagus to the rectum, serve distinct digestive functions. Some organs, such as the esophagus and glandular stomach or the small bowel and colon, are separated by sharp boundaries. The duodenal, jejunal and ileal segments of the small intestine, by contrast, have imprecise borders. Because human esophageal and gastric cancers frequently arise in a background of tissue metaplasia and some intestinal disorders are confined to discrete regions, it is useful to appreciate the molecular and cellular basis of boundary formation and preservation. Here we review the anatomy and determinants of boundaries and transitions in the alimentary canal with respect to tissue morphology, gene expression, and, especially, transcriptional control of epithelial identity. We discuss the evidence for established and candidate molecular mechanisms of boundary formation, including the solitary and combinatorial actions of tissue-restricted transcription factors. Although the understanding remains sparse, genetic studies in mice do provide insights into dominant mechanisms and point the way for future investigation.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
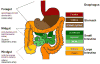
Figure 1. Organization of regions in the gastrointestinal tract
Left: The foregut, midgut and hindgut are classically defined according to the blood supply, as indicated. Right: Colors in the diagram refer to distinctive epithelia within the alimentary canal. In mice, the stratified squamous epithelium of the esophagus (crimson) extends into the dome-like forestomach, before forming a sharp, single-cell boundary with the glandular columnar epithelium of the gastric corpus (dark brown). As discussed in the text, the boundary between the gastric body and antral-pyloric epithelia (light brown) is less distinct, while that between the stomach and small intestine (green), marked by the pyloric sphincter, is sharp. Small intestine regions have different digestive functions and characteristic patterns of genes expression, without well-defined boundaries. The villous epithelium of small intestine transitions abruptly into a flat, non-villous epithelium at the ileo-cecal valve, which is followed by a specialized cecum (yellow) and the remainder of the colon (orange).
Figure 2. Expression domains of transcription factors that participate in digestive tissue identity and boundary formation
Left: Schematic diagram of the alimentary canal, stretched in the rostro-caudal axis, with discrete regions represented in the same colors as in Figure 1. Center: Expression domains of factors with known functions in epithelial specification or boundary formation. Factors expressed in the mesenchyme and epithelium are represented in black and gray, respectively. The many _Hox_-cluster genes expressed in the gut are not represented; see [39, 42, 48] for Hox gene expression. Right: Histologic demonstration of sharp anatomic boundaries at the mouse foregut squamo-columnar junction (top, H&E stain (adult)), gastroduodenal boundary (middle, H&E stain (adult), CDX2 immunohistochemistry (E16)), and ileo-cecal valve (bottom, Periodic acid Schiff stain (adult)). Arrows point to the respective junctions. At the foregut squamo-columnar junction, note the eosinophilic keratin lining the squamous epithelium, above the arrow, and its absence over glandular epithelium below the arrow.
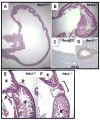
Figure 3. Illustrative boundary defects in mutant mice
(A-D) Hematoxylin and eosin (H&E)-stained neonatal stomach tissues from wild-type (A) and Barx1-/- (B) mice [52]. The homeotic posteriorization in Barx1-/- stomach is evident from ectopic presence of intestinal villi (B) and expression of the intestinal marker CDX2 (D) as early as E12.5 [27]. (E, F) H&E-stained wild-type (E) and Pdx1-/- (F) tissues at E18.5 reveal defective pylorus development in Pdx1-/- mice. Normally the stomach (s) opens into the duodenum (d) at the pylorus (p), where well-defined Brunner’s glands (b) are found. In Pdx1-/- mutants, a cavity that lacks villi and is lined by a cuboidal epithelium forms in this region [23].
Similar articles
- Macro- and microscopic anatomy of the digestive tract in the red-eared slider (Emydidae: Trachemys scripta elegans).
Miyai N, Kozono T, Kuriki T, Todoroki M, Murakami T, Shinohara K, Yoshida T, Kigata T. Miyai N, et al. PLoS One. 2024 Dec 30;19(12):e0315737. doi: 10.1371/journal.pone.0315737. eCollection 2024. PLoS One. 2024. PMID: 39774418 Free PMC article. - Histological and histochemical analysis of the gastrointestinal tract of the common pipistrelle bat (Pipistrellus pipistrellus).
Strobel S, Encarnação JA, Becker NI, Trenczek TE. Strobel S, et al. Eur J Histochem. 2015 Apr 13;59(2):2477. doi: 10.4081/ejh.2015.2477. Eur J Histochem. 2015. PMID: 26150154 Free PMC article. - Patterning the gastrointestinal epithelium to confer regional-specific functions.
Thompson CA, DeLaForest A, Battle MA. Thompson CA, et al. Dev Biol. 2018 Mar 15;435(2):97-108. doi: 10.1016/j.ydbio.2018.01.006. Epub 2018 Jan 12. Dev Biol. 2018. PMID: 29339095 Free PMC article. Review. - Signals and forces shaping organogenesis of the small intestine.
Wang S, Walton KD, Gumucio DL. Wang S, et al. Curr Top Dev Biol. 2019;132:31-65. doi: 10.1016/bs.ctdb.2018.12.001. Epub 2019 Jan 2. Curr Top Dev Biol. 2019. PMID: 30797512 Review.
Cited by
- Tissue Engineering for Gastrointestinal and Genitourinary Tracts.
Elia E, Brownell D, Chabaud S, Bolduc S. Elia E, et al. Int J Mol Sci. 2022 Dec 20;24(1):9. doi: 10.3390/ijms24010009. Int J Mol Sci. 2022. PMID: 36613452 Free PMC article. Review. - Stomach development, stem cells and disease.
Kim TH, Shivdasani RA. Kim TH, et al. Development. 2016 Feb 15;143(4):554-65. doi: 10.1242/dev.124891. Development. 2016. PMID: 26884394 Free PMC article. Review. - Gut Microbiome Transplants and Their Health Impacts across Species.
Levine BH, Hoffman JM. Levine BH, et al. Microorganisms. 2023 Jun 3;11(6):1488. doi: 10.3390/microorganisms11061488. Microorganisms. 2023. PMID: 37374992 Free PMC article. Review. - Functional physiology of the human terminal antrum defined by high-resolution electrical mapping and computational modeling.
Berry R, Miyagawa T, Paskaranandavadivel N, Du P, Angeli TR, Trew ML, Windsor JA, Imai Y, O'Grady G, Cheng LK. Berry R, et al. Am J Physiol Gastrointest Liver Physiol. 2016 Nov 1;311(5):G895-G902. doi: 10.1152/ajpgi.00255.2016. Epub 2016 Sep 22. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 27659422 Free PMC article. - De-repression of the RAC activator ELMO1 in cancer stem cells drives progression of TGFβ-deficient squamous cell carcinoma from transition zones.
McCauley HA, Chevrier V, Birnbaum D, Guasch G. McCauley HA, et al. Elife. 2017 Feb 21;6:e22914. doi: 10.7554/eLife.22914. Elife. 2017. PMID: 28219480 Free PMC article.
References
- Badreddine RJ, Wang KK. Barrett esophagus: an update. Nat Rev Gastroenterol Hepatol. 2010;7:369–378. - PubMed
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Beghtel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. - PubMed
- Comelli EM, Lariani S, Zwahlen MC, Fotopoulos G, Holzwarth JA, Cherbut C, Dorta G, Corthesy-Theulaz I, Grigorov M. Biomarkers of human gastrointestinal tract regions. Mamm Genome. 2009;20:516–527. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources