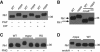A Drosophila metallophosphoesterase mediates deglycosylation of rhodopsin - PubMed (original) (raw)
A Drosophila metallophosphoesterase mediates deglycosylation of rhodopsin
Jinguo Cao et al. EMBO J. 2011.
Abstract
Oligosaccharide chains of newly synthesized membrane receptors are trimmed and modified to optimize their trafficking and/or signalling before delivery to the cell surface. For most membrane receptors, the functional significance of oligosaccharide chain modification is unknown. During the maturation of Rh1 rhodopsin, a Drosophila light receptor, the oligosaccharide chain is trimmed extensively. Neither the functional significance of this modification nor the enzymes mediating this process are known. Here, we identify a dmppe (Drosophila metallophosphoesterase) mutant with incomplete deglycosylation of Rh1, and show that the retained oligosaccharide chain does not affect Rh1 localization or signalling. The incomplete deglycosylation, however, renders Rh1 more sensitive to endocytic degradation, and causes morphological and functional defects in photoreceptors of aged dmppe flies. We further demonstrate that the dMPPE protein functions as an Mn(2+)/Zn(2+)-dependent phosphoesterase and mediates in vivo dephosphorylation of α-Man-II. Most importantly, the dephosphorylated α-Man-II is required for the removal of the Rh1 oligosaccharide chain. These observations suggest that the glycosylation status of membrane proteins is controlled through phosphorylation/dephosphorylation, and that MPPE acts as the phosphoesterase in this regulation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
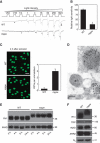
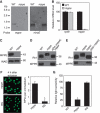
Newly eclosed dmppe mutants show reduced light sensitivity, abnormal distribution and high molecular weight of Rh1. (A) ERG recordings revealed the reduction of light sensitivity in newly eclosed dmppe mutants. Flies were raised in the dark and examined within 4 h after eclosion. Fly eyes were stimulated with a series of 1 s light pulses of increasing intensities as labelled on the top. The first response appearing is marked with an asterisk. The scale bar next to the top trace is 5 mV. (B) Quantification of light sensitivities in newly eclosed flies. The mean relative sensitivities shown were calculated as described in Materials and methods. The error bar represents standard error of the mean (s.e.m.). *Indicates that the sample is significantly different from others in the group. (C) Rh1 distribution in the photoreceptors of newly eclosed flies. Cross-sections were prepared as described in Materials and methods. The sections were stained with a monoclonal Rh1 antibody (4C5). The number of RPVs per ommatidium was calculated for each genotype. Scale bar, 5 μm. (D) Immunogold electron microscopy reveals that RPV appeared to be the aggregation of small vesicles. Sections were prepared as described in Materials and methods. One RPV (boxed) is enlarged in the upper panel. Scale bar, 2 μm. (E) Reduction of Rh1 level in newly eclosed dmppe mutants. Flies were raised in the dark and heads were collected at the indicated time after eclosion. The scaffold protein INAD was probed in parallel. (F) Western blots show the increase in Rh1 MW in the mutant. The MWs of other visual molecules are normal. WT: wild type.
Figure 2
The increase in Rh1 MW is due to a failure of deglycosylation. (A) The increase in Rh1 MW is not caused by phosphorylation modification. After digestion with calf intestinal phosphatase (CIP) or potato acid phosphatase (PAP) at 37°C for 16 h, purified Rh1 was subjected to SDS–PAGE and then immunoblotting. (B) Deletion of phosphorylation sites in Rh1 did not prevent the increase in Rh1 MW in the mutant background. Each lane was loaded with one fly head and probed with a polyclonal antibody against the N terminus of Rh1. Note: in the ninaE_Δ_356 allele, only truncated Rh1 can be detected, as this mutant allele was crossed into a ninaE null mutant background. (C) Rh1 bands shifted after the treatment with PNGase F. After digestion with PNGase F in 37°C for 16 h, purified Rh1 was subjected to SDS–PAGE and immunoblotting. RS: rescuing flies, dmppe; p[trp∷dmppe]. +: Incubation with PNGase F. −: Incubation without PNGase F. (D) The MW of Rh1 was reduced after the treatment with Endoglycosidase H. Purified Rh1 was incubated with Endoglycosidase H at 37°C for 16 h and examined by western blots.
Figure 3
Deglycosylation of Rh1 is essential for transport, but not required for membrane localization. (A) Time course of Rh1 processing in wild-type flies. Pupal heads were collected at the indicated time. The amount loaded is labelled on the top. (B) The Rh1 deglycosylation process is disrupted in dmppe mutant. Pupae were collected at different pupal development time points. (C) Rh1 distribution in the developing photoreceptors of wild-type flies. Sections were prepared as described in Materials and methods. Cross-sections were stained with anti-Rh1 antibody (green) and rhodamine-phalloidin (red). % pd: % of pupal development. Scale bar, 5 μm. (D) Rh1 distribution in the developing photoreceptors of the dmppe mutant. Scale bar, 5 μm. (E) Quantification of the percentage of Rh1 in rhabdomere during pupal development. Quantification was performed as described in Materials and methods. (F) Most Rh1 localized normally in matured mutant photoreceptors. Flies were raised in the dark and examined at 2 days after eclosion. Collection and fixation were performed under dim red light. Scale bar, 5 μm. (G) Constant Rh1 levels in adult mutants. Flies were raised in the dark and examined at the indicated time after eclosion. The scaffold protein INAD was probed in parallel as a loading control.
Figure 4
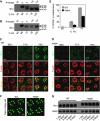
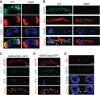
Glycosylated Rh1 is not stable once it is endocytosed, and leads to retinal degeneration. (A) Quantification of light sensitivities in wild-type and dmppe mutant flies. Flies were raised in 12 h light/12 h dark conditions. Newly eclosed adults were collected and reared for the indicated time. For 1-day-old flies, collected adults were reared for 24 h. Relative light sensitivity was measured and calculated as described in Materials and methods. (B) Rh1 and INAD levels decreased in the older mutant. Tubulin was probed as a loading control. The reduction of INAD level indicates the older mutant undergoes retinal degeneration. (C) EM analyses revealed the older mutant underwent retinal degeneration. Flies were raised in 12 h light/12 h dark conditions for the indicated time. Each picture shows a single ommatidium. One degenerated rhabdomere from the mutant is enlarged in the right panel. Scale bar, 2 μm. (D) Glycosylation does not affect light-induced endocytosis of Rh1. Two-day-old dark-reared flies were stimulated with white light (700 lux) for 8 h and stained with the monoclonal Rh1 antibody. Scale bar, 5 μm. (E) Rh1 distribution after blue light stimulation. Two-day-old, dark-reared flies were stimulated with pure blue light (700 lux) for 2 or 3 h. Cross-sections were prepared and stained as described in Materials and methods. Three large endocytic Rh1 particles in the cell bodies are marked with arrows. Scale bar, 5 μm. The right panel shows the ratio of Rh1 signal intensity in the cell body for each genotype and treatment. Quantification was performed as described in Materials and methods. (F) Rh1 level decreased in the mutant after 3 h under blue light stimulation. Two-day-old, dark-reared flies were stimulated with pure blue light (700 lux) for the indicated time. After stimulation, fly heads were collected and total Rh1 levels were compared by western blots. INAD was probed as a loading control. *indicates that the sample is significantly different from the control (P<0.01; Student's _t_-test).
Figure 5
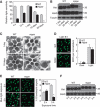
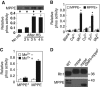
dMPPE is required for Rh1 deglycosylation. (A) Northern blot shows the mRNA of the dmppe gene is absent in dmppe mutants. Total RNA was isolated from the heads of <3-day-old cn,bw and cn,pBacCG8889 e02905 ,bw flies using Trizol. Total RNA (10 μg) was subjected to 1% denaturing agarose gels and hydrated with a DIG-labelled dmppe cDNA probe. The membrane was stripped and re-probed with ninaE probe for the loading control. (B) Real-time RT–PCR reveals that the dmppe mRNA level decreases ∼50-fold in dmppe mutants. Three data sets were averaged. (C) Western blots showing loss of dMPPE protein in the mutant. Each lane was loaded with three fly heads. (D) Overexpression of dMPPE restored the MW of Rh1. cn, mppe,bw;p[hs∷mppe] flies were heat shocked every day for 1 h from early pupal stage. Newly eclosed adults were collected for western blots. dMPPE protein levels are showed on the lower panel. (E) Photoreceptor-specific expression of dMPPE restored the MW of Rh1. dMPPE was specifically expressed in the photoreceptor through a trp gene promoter. dMPPE protein levels are shown on the lower panel. (F) Rh1 distribution in newly eclosed rescue (RS) flies. Quantifications of RPV number per ommatidium are presented in the right panel. The RS represents cn, mppe,bw; p[trp∷mppe]/TM6c flies. Scale bar, 5 μm. (G) Light sensitivity was restored in the RS fly. The relative sensitivities were calculated as described in Materials and methods. *indicates that the sample is significantly different from the control (P<0.01; Student's _t_-test).
Figure 6
dMPPE localizes in Golgi and colocalizes with trafficking Rh1. (A) dMPPE is predominately expressed in the retina. Dissected fly heads were costained with dMPPE antibody (green), a 24b10 antibody (red, showing retina) and DAPI (blue, for nuclei). R=retina, L=lamina, M=medulla, B=brain. Scale bar, 200 μm. (B) dMPPE distribution in photoreceptors. Dissected ommatidia were costained with dMPPE antibody (green), an Rh1 antibody (red, showing rhabdomere bundles) and DAPI (blue, for nuclei). Scale bar, 10 μm. (C) In GMR-Gal4/p[UAS∷GFP-KDEL] flies, dMPPE does not colocalize with GFP-KEDL. Dissected ommatidia were costained with dMPPE antibody (red), GFP antibody (green, showing ER) and TO-PRO-3 iodide (blue, for nuclei). Scale bar, 10 μm. (D) In GMR-Gal4/p[UAS∷galactosyltransferase-GFP] flies, dMPPE colocalizes with the GFP signal. Dissected ommatidia were costained with dMPPE antibody (red), GFP antibody (green, showing Golgi) and TO-PRO-3 iodide (blue, for nuclei). Scale bar, 10 μm. (E) dMPPE colocalizes with trafficking Rh1 vesicles (arrows). At 68% pd, pupae of wild-type flies were collected. Sections were costained with Rh1 antibody (red), dMPPE antibody (green) and phalloidin (blue, showing rhabdomere bundles). Scale bar, 5 μm.
Figure 7
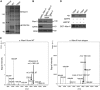
dMPPE functions as a phosphoesterase. (A) Time courses of dMPPE expression (top, western blot) and phosphoesterase activity in fly head extracts (bottom). After heat shock at 37°C for 1 h, the heads of p[hs∷mppe] transgenic flies were collected at different time points. The protein levels of dMPPE and total phosphoesterase activity of hydrolysed p-nitrophenyl phosphate were measured. The mean values and s.e.m. of three sets of data are presented. (B) Bacterially expressed dMPPE protein displays metal-dependent phosphoesterase activity. His-tagged dMPPE protein was expressed in E. coli and purified from cell lysate using an Ni2+ affinity column. The phosphoesterase activity was measured by hydrolysis of p-nitrophenyl phosphate (pNpp). After averaging three sets of data, the mean values of phosphoesterase activity and s.e.m. are presented. (C) Phosphoesterase activity was abolished in truncated dMPPEΔ protein, which deleted amino acid 164–183. After averaging three sets of data, the mean of phosphoesterase activity and s.e.m. are presented. (D) The phosphoesterase activity of dMPPE is essential for Rh1 deglycosylation. Eye-specific expression of truncated dMPPEΔ cannot restore the MW of Rh1. dMPPE protein and truncated dMPPEΔ protein levels are shown. *indicates that the sample is significantly different from the control (P<0.01; Student's _t_-test).
Figure 8
dMPPE directly dephosphorylates α-Man-II. (A) Identification of phosphorylated proteins in dmppe mutants. Phosphorylated protein enrichment was performed as described in Materials and methods. Bands whose amount increased in the dmppe mutant sample were cut and identified by MS. Detailed results from the MS analysis are provided in Supplementary data. (B) Ectopic expression of α-Man-II in wild-type and dmppe mutant flies. (C) Identification of the phosphorylated peptide from the dmppe mutant. Myc-tagged α-Man-II was immunoprecipitated from wild-type and mutant flies. Trypsin-digested peptides were used for MS analysis. In the dmppe mutant sample, the m/z 1821.97 ion is lost with the appearance of an m/z 1901.94 ion. (D) dMPPE directly dephosphorylates α-Man-II in vitro. Phosphorylation and dephosphorylation reactions were performed as described in Materials and methods. Upper panel: Autoradiogram of SDS–PAGE of 32P in vitro labelled GST-α-Man-II recombinant protein. Lower panel: The same gel blotted and probed with antibodies to GST. rMPPE represents purified recombinant dMPPE and rMPPEΔ represents purified recombinant truncated dMPPE.
Figure 9
α-Man-II mediates Rh1 deglycosylation in vivo. (A) The MW of Rh1 was reduced after treatment with α1-6 mannosidase or α1-2,3 mannosidase. Purified Rh1 was incubated with different glycosidases at 37°C for 16 h. (B) The MW of Rh1 was reduced after digestion with recombinant α-Man-II. Recombinant dMPPE and GST-α-Man-II were purified as described in Materials and methods. Purified Rh1 was incubated with GST–α-Man-II alone or with the mixture of GST–α-Man-II and dMPPE in 37°C for 16 h. rMPPE represents purified recombinant dMPPE and rMan-II represents purified recombinant GST–α-Man-II. Note that the activity of recombinant α-Man-II does not depend on the presence of dMPPE. (C) The MW of Rh1 from the α_-Man-II_ mutant was similar to that of the dmppe mutant. (D) The MW of Rh1 from α_-Man-II_ mutant was reduced after digestion with PNGase F. (E) Expression of the mutant form α-Man-IIS73G, but not wild-type α-Man-II, restored the MW of Rh1 in the dmppe mutant background. α-Man-II (Myc) and dMPPE expression levels are shown. (F) The role of dMPPE in Rh1 deglycosylation. dMPPE directly dephosphorylates α-Man-II, which in turn removes two mannoses from the oligosaccharide chain of Rh1. N-acetylglucosamine residues are shown as circles and mannose residues are shown as squares.
Similar articles
- Metallophosphoesterase regulates light-induced rhodopsin endocytosis by promoting an association between arrestin and the adaptor protein AP2.
Mu Y, Tian Y, Zhang ZC, Han J. Mu Y, et al. J Biol Chem. 2019 Aug 30;294(35):12892-12900. doi: 10.1074/jbc.RA119.009602. Epub 2019 Jul 19. J Biol Chem. 2019. PMID: 31324721 Free PMC article. - Mutations in four glycosyl hydrolases reveal a highly coordinated pathway for rhodopsin biosynthesis and N-glycan trimming in Drosophila melanogaster.
Rosenbaum EE, Vasiljevic E, Brehm KS, Colley NJ. Rosenbaum EE, et al. PLoS Genet. 2014 May 1;10(5):e1004349. doi: 10.1371/journal.pgen.1004349. eCollection 2014 May. PLoS Genet. 2014. PMID: 24785692 Free PMC article. - Drosophila fabp is required for light-dependent Rhodopsin-1 clearance and photoreceptor survival.
Huang HW, Ryoo HD. Huang HW, et al. PLoS Genet. 2021 Oct 29;17(10):e1009551. doi: 10.1371/journal.pgen.1009551. eCollection 2021 Oct. PLoS Genet. 2021. PMID: 34714826 Free PMC article. - Molecular genetics of retinal degeneration: A Drosophila perspective.
Shieh BH. Shieh BH. Fly (Austin). 2011 Oct-Dec;5(4):356-68. doi: 10.4161/fly.5.4.17809. Epub 2011 Sep 7. Fly (Austin). 2011. PMID: 21897116 Free PMC article. Review. - Membrane protein trafficking in Drosophila photoreceptor cells.
Schopf K, Huber A. Schopf K, et al. Eur J Cell Biol. 2017 Aug;96(5):391-401. doi: 10.1016/j.ejcb.2016.11.002. Epub 2016 Dec 7. Eur J Cell Biol. 2017. PMID: 27964885 Review.
Cited by
- Phosphorylation of the Drosophila transient receptor potential ion channel is regulated by the phototransduction cascade and involves several protein kinases and phosphatases.
Voolstra O, Bartels JP, Oberegelsbacher C, Pfannstiel J, Huber A. Voolstra O, et al. PLoS One. 2013 Sep 9;8(9):e73787. doi: 10.1371/journal.pone.0073787. eCollection 2013. PLoS One. 2013. PMID: 24040070 Free PMC article. - The N's and O's of Drosophila glycoprotein glycobiology.
Katoh T, Tiemeyer M. Katoh T, et al. Glycoconj J. 2013 Jan;30(1):57-66. doi: 10.1007/s10719-012-9442-x. Epub 2012 Aug 31. Glycoconj J. 2013. PMID: 22936173 Free PMC article. Review. - Phenotype-based clustering of glycosylation-related genes by RNAi-mediated gene silencing.
Yamamoto-Hino M, Yoshida H, Ichimiya T, Sakamura S, Maeda M, Kimura Y, Sasaki N, Aoki-Kinoshita KF, Kinoshita-Toyoda A, Toyoda H, Ueda R, Nishihara S, Goto S. Yamamoto-Hino M, et al. Genes Cells. 2015 Jun;20(6):521-42. doi: 10.1111/gtc.12246. Epub 2015 May 4. Genes Cells. 2015. PMID: 25940448 Free PMC article. - Drosophila neuroligin 4 regulates sleep through modulating GABA transmission.
Li Y, Zhou Z, Zhang X, Tong H, Li P, Zhang ZC, Jia Z, Xie W, Han J. Li Y, et al. J Neurosci. 2013 Sep 25;33(39):15545-54. doi: 10.1523/JNEUROSCI.0819-13.2013. J Neurosci. 2013. PMID: 24068821 Free PMC article. - Phosphatidic acid phospholipase A1 mediates ER-Golgi transit of a family of G protein-coupled receptors.
Kunduri G, Yuan C, Parthibane V, Nyswaner KM, Kanwar R, Nagashima K, Britt SG, Mehta N, Kotu V, Porterfield M, Tiemeyer M, Dolph PJ, Acharya U, Acharya JK. Kunduri G, et al. J Cell Biol. 2014 Jul 7;206(1):79-95. doi: 10.1083/jcb.201405020. J Cell Biol. 2014. PMID: 25002678 Free PMC article.
References
- Brown G, Chen DM, Christianson JS, Lee R, Stark WS (1994) Receptor demise from alteration of glycosylation site in Drosophila opsin: electrophysiology, microspectrophotometry, and electron microscopy. Vis Neurosci 11: 619–628 - PubMed
- Chen S, Yakunin AF, Kuznetsova E, Busso D, Pufan R, Proudfoot M, Kim R, Kim SH (2004) Structural and functional characterization of a novel phosphodiesterase from Methanococcus jannaschii. J Biol Chem 279: 31854–31862 - PubMed
- Chmelar RS, Nathanson NM (2006) Identification of a novel apical sorting motif and mechanism of targeting of the M2 muscarinic acetylcholine receptor. J Biol Chem 281: 35381–35396 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous