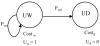A Markov model to analyze cost-effectiveness of screening for severe combined immunodeficiency (SCID) - PubMed (original) (raw)
A Markov model to analyze cost-effectiveness of screening for severe combined immunodeficiency (SCID)
Kee Chan et al. Mol Genet Metab. 2011 Nov.
Abstract
Objective: To evaluate the cost-effectiveness of universal neonatal screening for T cell lymphocytopenia in enhancing quality of life and life expectancy for children with severe combined immunodeficiency (SCID).
Methods: Decision trees were created and analyzed to estimate the cost, life years, and quality adjusted life years (QALYs) across a population when universal screening for lack of T cells is used to detect SCID, as implemented in five states, compared to detection based on recognizing symptoms and signs of disease. Terminal values of each tree limb were derived through Markov models simulating the natural history of three cohorts: unaffected subjects; those diagnosed with SCID as neonates (early diagnosis); and those diagnosed after becoming symptomatic and arousing clinical suspicion (late diagnosis). Models considered the costs of screening and of care including hematopoietic cell transplantation for affected individuals. Key decision variables were derived from the literature and from a survey of families with children affected by SCID, which was used to describe the clinical history and healthcare utilization for affected subjects. Sensitivity analyses were conducted to explore the influence of these decision variables.
Results: Over a 70-year time horizon, the average cost per infant was 8.89withoutscreeningand8.89 without screening and 8.89withoutscreeningand14.33 with universal screening. The model predicted that universal screening in the U.S. would cost approximately 22.4million/yearwithagainof880lifeyearsand802QALYs.Sensitivityanalysesshowedthatscreeningtestspecificityanddiseaseincidencewerecriticaldrivingforcesaffectingtheincrementalcost−effectivenessratio(ICER).AssumingaSCIDincidenceof1/75,000birthsandtestspecificityandsensitivityeachat0.99,screeningremainedcost−effectiveuptoamaximumcostof22.4 million/year with a gain of 880 life years and 802 QALYs. Sensitivity analyses showed that screening test specificity and disease incidence were critical driving forces affecting the incremental cost-effectiveness ratio (ICER). Assuming a SCID incidence of 1/75,000 births and test specificity and sensitivity each at 0.99, screening remained cost-effective up to a maximum cost of 22.4million/yearwithagainof880lifeyearsand802QALYs.Sensitivityanalysesshowedthatscreeningtestspecificityanddiseaseincidencewerecriticaldrivingforcesaffectingtheincrementalcost−effectivenessratio(ICER).AssumingaSCIDincidenceof1/75,000birthsandtestspecificityandsensitivityeachat0.99,screeningremainedcost−effectiveuptoamaximumcostof15 per infant screened.
Conclusion: At our current estimated screening cost of $4.22/infant, universal screening for SCID would be a cost effective means to improve quality and duration of life for children with SCID.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
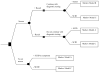
Figure 1
Markov model analytic decision tree comparing two strategies: 1) newborn screening, and 2) no screening. The following models at the terminal end of each limb depict the possible stochastic processes of affected SCID identified early by screening (Markov model B), affected SCID identified after manifestation of symptoms (Markov model A), or unaffected non-SCID infants (Markov model C).
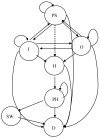
Figure 2
Transition state Markov model A and B: Both models have all the same health states,; In Model B, one additional transition is allowed as shown by the dotted arrow to demonstrate moving from presymptomatic SCID directly to HCT following screening. Abbreviations as in Table 1.
Figure 3
Transition state Markov model of two states of an unaffected infant (Markov model C).
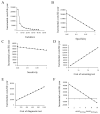
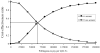
Figure 4
One-way sensitivity analysis of incremental cost per quality-adjust life year (QALYs) as function of A) prevalence, B) test sensitivity, C) test specificity, D) cost of screening test and E) cost of confirmation and diagnostic testing; and F) the ratio of cost of BMTlate vs. BMTearly (cost BMTlate/cost BMTearly).
Figure 5
Ranges of uncertainty given at a range of different willingness to pay per QALY is illustrated by a cost-effectiveness acceptability curve. Estimates for the variables were varied from a distribution at random for incidence, cost of screening test, sensitivity and specificity. Dotted line indicates the cross-point of the willingness to pay per QALY at 0.50 cost effective.
Similar articles
- Systematic neonatal screening for severe combined immunodeficiency and severe T-cell lymphopenia: Analysis of cost-effectiveness based on French real field data.
Clément MC, Mahlaoui N, Mignot C, Le Bihan C, Rabetrano H, Hoang L, Neven B, Moshous D, Cavazzana M, Blanche S, Fischer A, Audrain M, Durand-Zaleski I. Clément MC, et al. J Allergy Clin Immunol. 2015 Jun;135(6):1589-93. doi: 10.1016/j.jaci.2015.02.004. Epub 2015 Apr 1. J Allergy Clin Immunol. 2015. PMID: 25840725 - The case for severe combined immunodeficiency (SCID) and T cell lymphopenia newborn screening: saving lives…one at a time.
Quinn J, Orange JS, Modell V, Modell F. Quinn J, et al. Immunol Res. 2020 Feb;68(1):48-53. doi: 10.1007/s12026-020-09117-9. Immunol Res. 2020. PMID: 32128663 - Potential costs and benefits of newborn screening for severe combined immunodeficiency.
McGhee SA, Stiehm ER, McCabe ER. McGhee SA, et al. J Pediatr. 2005 Nov;147(5):603-8. doi: 10.1016/j.jpeds.2005.06.001. J Pediatr. 2005. PMID: 16291349 - Newborn screening for severe combined immune deficiency (technical and political aspects).
Kobrynski L. Kobrynski L. Curr Opin Allergy Clin Immunol. 2015 Dec;15(6):539-46. doi: 10.1097/ACI.0000000000000221. Curr Opin Allergy Clin Immunol. 2015. PMID: 26485096 Review. - History and current status of newborn screening for severe combined immunodeficiency.
Kwan A, Puck JM. Kwan A, et al. Semin Perinatol. 2015 Apr;39(3):194-205. doi: 10.1053/j.semperi.2015.03.004. Epub 2015 Apr 30. Semin Perinatol. 2015. PMID: 25937517 Free PMC article. Review.
Cited by
- TREC Based Newborn Screening for Severe Combined Immunodeficiency Disease: A Systematic Review.
van der Spek J, Groenwold RH, van der Burg M, van Montfrans JM. van der Spek J, et al. J Clin Immunol. 2015 May;35(4):416-30. doi: 10.1007/s10875-015-0152-6. Epub 2015 Apr 17. J Clin Immunol. 2015. PMID: 25893636 Free PMC article. Review. - Newborn blood spot screening test using multiplexed real-time PCR to simultaneously screen for spinal muscular atrophy and severe combined immunodeficiency.
Taylor JL, Lee FK, Yazdanpanah GK, Staropoli JF, Liu M, Carulli JP, Sun C, Dobrowolski SF, Hannon WH, Vogt RF. Taylor JL, et al. Clin Chem. 2015 Feb;61(2):412-9. doi: 10.1373/clinchem.2014.231019. Epub 2014 Dec 11. Clin Chem. 2015. PMID: 25502182 Free PMC article. - Modern diagnostic capabilities of neonatal screening for primary immunodeficiencies in newborns.
Khalturina EO, Degtyareva ND, Bairashevskaia AV, Mulenkova AV, Degtyareva AV. Khalturina EO, et al. Clin Exp Pediatr. 2021 Oct;64(10):504-510. doi: 10.3345/cep.2020.01270. Epub 2021 Mar 25. Clin Exp Pediatr. 2021. PMID: 33781055 Free PMC article. - Severe Combined Immunodeficiency (SCID) and Its New Treatment Modalities.
Wadbudhe AM, Meshram RJ, Tidke SC. Wadbudhe AM, et al. Cureus. 2023 Oct 26;15(10):e47759. doi: 10.7759/cureus.47759. eCollection 2023 Oct. Cureus. 2023. PMID: 38022338 Free PMC article. Review.
References
- Buckley RH. Primary cellular immunodeficiencies. Journal of Allergy & Clinical Immunology. 2002;109:747–757. - PubMed
- Campbell E, Ross LF. Parental attitudes regarding newborn screening of PKU and DMD. American Journal of Medical Genetics. Part A. 2003;120:209–214. - PubMed
- Howell RR. Introduction: newborn screening. Ment Retard Dev Disabil Res Rev. 2006;12:229. - PubMed
- Brown L, Xu-Bayford J, Allwood Z, Slatter M, Cant A, Davies EG, Veys P, Gennery AR, Gaspar HB. Neonatal diagnosis of severe combined immunodeficiency leads to significantly improved survival outcome: the case for newborn screening. Blood. 2011;117:3243–3246. - PubMed
- Buckley RH. Advances in the understanding and treatment of human severe combined immunodeficiency. Immunologic Research. 2000;22:237–251. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical