Understanding the role of TDP-43 and FUS/TLS in ALS and beyond - PubMed (original) (raw)
Review
Understanding the role of TDP-43 and FUS/TLS in ALS and beyond
Sandrine Da Cruz et al. Curr Opin Neurobiol. 2011 Dec.
Abstract
Dominant mutations in two DNA/RNA binding proteins, TDP-43 and FUS/TLS, are causes of inherited Amyotrophic Lateral Sclerosis (ALS). TDP-43 and FUS/TLS have striking structural and functional similarities, implicating alterations in RNA processing as central in ALS. TDP-43 has binding sites within a third of all mouse and human mRNAs in brain and this binding influences the levels and splicing patterns of at least 20% of those mRNAs. Disease modeling in rodents of the first known cause of inherited ALS-mutation in the ubiquitously expressed superoxide dismutase (SOD1)-has yielded non-cell autonomous fatal motor neuron disease caused by one or more toxic properties acquired by the mutant proteins. In contrast, initial disease modeling for TDP-43 and FUS/TLS has produced highly varied phenotypes. It remains unsettled whether TDP-43 and FUS/TLS mutants provoke disease from a loss of function or gain of toxicity or both. TDP-43 or FUS/TLS misaccumulation seems central not just to ALS (where it is found in almost all instances of disease), but more broadly in neurodegenerative disease, including frontal temporal lobular dementia (FTLD-U) and many examples of Alzheimer's or Huntington's disease.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
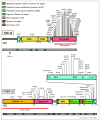
Figure 1. TDP-43 and FUS/TLS mutations in ALS and FTLD patients
(Upper panel) Forty-four mutations have been identified in TDP-43 in sporadic and familial ALS patients and in rare FTLD patients, with most lying in the C-terminal glycine-rich region. The putative prion domain comprises amino acids from 277–414. (Lower panel) Forty-three mutations have been identified in FUS/TLS in familial and sporadic ALS cases and in rare FTLD patients. Most mutations are clustered in the last 17 amino acids and in the glycine-rich region and the putative prion domain comprises amino acids 1–239.
Similar articles
- Divergent roles of ALS-linked proteins FUS/TLS and TDP-43 intersect in processing long pre-mRNAs.
Lagier-Tourenne C, Polymenidou M, Hutt KR, Vu AQ, Baughn M, Huelga SC, Clutario KM, Ling SC, Liang TY, Mazur C, Wancewicz E, Kim AS, Watt A, Freier S, Hicks GG, Donohue JP, Shiue L, Bennett CF, Ravits J, Cleveland DW, Yeo GW. Lagier-Tourenne C, et al. Nat Neurosci. 2012 Nov;15(11):1488-97. doi: 10.1038/nn.3230. Epub 2012 Sep 30. Nat Neurosci. 2012. PMID: 23023293 Free PMC article. - Rethinking ALS: the FUS about TDP-43.
Lagier-Tourenne C, Cleveland DW. Lagier-Tourenne C, et al. Cell. 2009 Mar 20;136(6):1001-4. doi: 10.1016/j.cell.2009.03.006. Cell. 2009. PMID: 19303844 Free PMC article. Review. - ALS-associated mutations in TDP-43 increase its stability and promote TDP-43 complexes with FUS/TLS.
Ling SC, Albuquerque CP, Han JS, Lagier-Tourenne C, Tokunaga S, Zhou H, Cleveland DW. Ling SC, et al. Proc Natl Acad Sci U S A. 2010 Jul 27;107(30):13318-23. doi: 10.1073/pnas.1008227107. Epub 2010 Jul 12. Proc Natl Acad Sci U S A. 2010. PMID: 20624952 Free PMC article. - Molecular basis of amyotrophic lateral sclerosis.
Liscic RM, Breljak D. Liscic RM, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2011 Mar 30;35(2):370-2. doi: 10.1016/j.pnpbp.2010.07.017. Epub 2010 Jul 23. Prog Neuropsychopharmacol Biol Psychiatry. 2011. PMID: 20655970 Review. - TDP-43/FUS in motor neuron disease: Complexity and challenges.
Guerrero EN, Wang H, Mitra J, Hegde PM, Stowell SE, Liachko NF, Kraemer BC, Garruto RM, Rao KS, Hegde ML. Guerrero EN, et al. Prog Neurobiol. 2016 Oct-Nov;145-146:78-97. doi: 10.1016/j.pneurobio.2016.09.004. Epub 2016 Sep 28. Prog Neurobiol. 2016. PMID: 27693252 Free PMC article. Review.
Cited by
- Transposable elements in TDP-43-mediated neurodegenerative disorders.
Li W, Jin Y, Prazak L, Hammell M, Dubnau J. Li W, et al. PLoS One. 2012;7(9):e44099. doi: 10.1371/journal.pone.0044099. Epub 2012 Sep 5. PLoS One. 2012. PMID: 22957047 Free PMC article. - Amyotrophic Lateral Sclerosis associated FUS mutation shortens mitochondria and induces neurotoxicity.
Nakaya T, Maragkakis M. Nakaya T, et al. Sci Rep. 2018 Oct 22;8(1):15575. doi: 10.1038/s41598-018-33964-0. Sci Rep. 2018. PMID: 30349096 Free PMC article. - Xrp1 genetically interacts with the ALS-associated FUS orthologue caz and mediates its toxicity.
Mallik M, Catinozzi M, Hug CB, Zhang L, Wagner M, Bussmann J, Bittern J, Mersmann S, Klämbt C, Drexler HCA, Huynen MA, Vaquerizas JM, Storkebaum E. Mallik M, et al. J Cell Biol. 2018 Nov 5;217(11):3947-3964. doi: 10.1083/jcb.201802151. Epub 2018 Sep 12. J Cell Biol. 2018. PMID: 30209068 Free PMC article. - Low complexity RGG-motif sequence is required for Processing body (P-body) disassembly.
Roy R, Das G, Kuttanda IA, Bhatter N, Rajyaguru PI. Roy R, et al. Nat Commun. 2022 Apr 19;13(1):2077. doi: 10.1038/s41467-022-29715-5. Nat Commun. 2022. PMID: 35440550 Free PMC article. - Genetics of amyotrophic lateral sclerosis: an update.
Chen S, Sayana P, Zhang X, Le W. Chen S, et al. Mol Neurodegener. 2013 Aug 13;8:28. doi: 10.1186/1750-1326-8-28. Mol Neurodegener. 2013. PMID: 23941283 Free PMC article. Review.
References
- Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. - PubMed
- Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. - PubMed
- Wang L, Gutmann DH, Roos RP. Astrocyte loss of mutant SOD1 delays ALS disease onset and progression in G85R transgenic mice. Hum Mol Genet. 2010;20:286–293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS27036/NS/NINDS NIH HHS/United States
- R01 NS027036/NS/NINDS NIH HHS/United States
- RC1 NS069144/NS/NINDS NIH HHS/United States
- 089701/WT_/Wellcome Trust/United Kingdom
- R37 NS027036/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous