Adult hippocampal neurogenesis buffers stress responses and depressive behaviour - PubMed (original) (raw)
Adult hippocampal neurogenesis buffers stress responses and depressive behaviour
Jason S Snyder et al. Nature. 2011.
Abstract
Glucocorticoids are released in response to stressful experiences and serve many beneficial homeostatic functions. However, dysregulation of glucocorticoids is associated with cognitive impairments and depressive illness. In the hippocampus, a brain region densely populated with receptors for stress hormones, stress and glucocorticoids strongly inhibit adult neurogenesis. Decreased neurogenesis has been implicated in the pathogenesis of anxiety and depression, but direct evidence for this role is lacking. Here we show that adult-born hippocampal neurons are required for normal expression of the endocrine and behavioural components of the stress response. Using either transgenic or radiation methods to inhibit adult neurogenesis specifically, we find that glucocorticoid levels are slower to recover after moderate stress and are less suppressed by dexamethasone in neurogenesis-deficient mice than intact mice, consistent with a role for the hippocampus in regulation of the hypothalamic-pituitary-adrenal (HPA) axis. Relative to controls, neurogenesis-deficient mice also showed increased food avoidance in a novel environment after acute stress, increased behavioural despair in the forced swim test, and decreased sucrose preference, a measure of anhedonia. These findings identify a small subset of neurons within the dentate gyrus that are critical for hippocampal negative control of the HPA axis and support a direct role for adult neurogenesis in depressive illness.
Figures
Figure 1
GFAP-TK mice show specific inhibition of adult neurogenesis. a) Confocal image of endogenous GFAP and transgenic TK expression in a radial precursor cell in the dentate gyrus (arrow). b) Confocal photographs of valganciclovir-treated mice show GFAP+ astrocytes in the hilus and molecular layer in both genotypes, despite strong TK expression in all GFAP-expressing cells in TK mice. c) Valganciclovir treatment did not affect numbers of GFAP+ astrocytes (genotype effect F1,20=1.7, P=0.2; valganciclovir effect F1,20=1.0, P=0.3; interaction F1,20=1.5, P=0.2; n=6/group), confirming the expectation that valganciclovir does not kill astrocytes, which are postmitotic in the adult. d) Confocal photographs of dentate gyrus doublecortin (DCX) immunostaining in mice treated with valganciclovir (v-WT and v-TK) for 4 weeks. DCX+ young neurons are abundant in v-WT mice but absent in v-TK mice. e) The number of BrdU+/DCX+ young neurons in was unaltered in v-WT mice but reduced by 99% in v-TK mice (genotype effect F1,20=20, P=0.0002; valganciclovir effect F1,20=19, P=0.0003; interaction F1,20=40, P<0.0001; *P<0.001 vs. untreated TK and v-WT; n=6/group). Inset shows example of 1-day-old BrdU+/DCX+ neuron (arrow). Error bars show s.e.m.; scale bars 10 μm in a, e and 100 μm in b, d. MOL, molecular layer; GCL, granule cell layer; HIL, hilus.
Figure 2
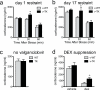
The glucocorticoid response to stress is increased in neurogenesis-deficient mice. a) Restraint, a moderate psychogenic stressor, resulted in higher corticosterone in neurogenesis-deficient v-TK mice than in v-WT mice 30 min after the end of stress. b) The effect of restraint was still observed after repeated exposure to stress (for both days: genotype effect F1,65>5, P<0.05; time effect F2,65>24, P<0.001; *P<0.05 post hoc vs. v-WT; n=6-17/group/timepoint). c) In untreated control mice, corticosterone levels 30 min after restraint stress were not different between WT and TK, indicating that altered glucocorticoid response to stress is not a non-specific effect of transgene expression (t30=0.1, P=0.95; n=16/group). d) Valganciclovir-treated v-TK mice show impaired dexamethasone suppression of corticosterone in response to restraint (*t13=2.5, P=0.03; n=7-8/group). Error bars show s.e.m.
Figure 3
Increased stress response is not due to reduced neurogenesis in the subventricular zone. a) Increased corticosterone response 30 min after restraint was confirmed in mice in which neurogenesis was reduced by irradiation (irradiation effect F1,50=2.0, P=0.16; time effect F2,50=5.1, P=0.01; irradiation × time interaction F2,50=5.7, P=0.006; *P<0.01 post hoc; n=4-6/group/time point). Green squares at the 30 min time point indicate corticosterone levels in irradiated mice that showed unaffected neurogenesis in the subventricular zone (SVZ). b) Confocal images of BrdU+ and DCX+ cells in the dentate gyrus; neurogenesis was reduced in all irradiated mice. c) Confocal images of BrdU+ and DCX+ cells illustrating sparing of neurogenesis in the SVZ. Scale bars 100 μm. LV, lateral ventricle; MOL, molecular layer; GCL, granule cell layer; HIL, hilus.
Figure 4
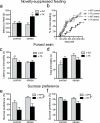
Mice lacking neurogenesis show increased anxiety/depression-like behaviors. a) In the novelty-suppressed feeding (NSF) test, v-TK mice showed increased latency to feed in a novel environment following restraint stress but not under control conditions (genotype effect F1,75=5.9, P=0.02; stress effect F1,75=1.9, P=0.17; genotype × stress interaction F1,75=4.8, P=0.03; *P<0.05 vs. v-TK control and *P<0.01 vs. v-WT stressed; n=13-25/group). b) Cumulative distribution of feeding latencies for the NSF test (log-rank test; *P<0.05 vs. all other groups). c) Neurogenesis-deficient v-TK mice became immobile faster in the forced swim test. Restraint stress reduced the latency to immobility in v-WT mice but did not affect v-TK mice (genotype effect F1,88=1.1, P=0.3; stress effect F1,88=2.2, P=0.14; genotype × stress interaction F1,88=2.6, P=0.11; *T46=2.1, P<0.05 vs. v-WT stressed; v-TK control vs. v-TK stressed T42=0.1, P=0.9; n=22-26/group). d) Under control conditions, the total time spent immobile was greater in v-TK mice than in v-WT mice. Restraint stress significantly increased total immobility in v-WT mice but had no effect on v-TK mice (genotype effect F1,88=0.3, P=0.6; stress effect F1,88=4.2, P=0.04; genotype × stress interaction F1,88=9.1, P=0.003; *P<0.05 vs. control v-TK, *P<0.001 vs. stressed v-WT, stressed v-WT vs. stressed v-TK P>0.05; n=22-26/group). e) Neurogenesis-deficient v-TK mice showed reduced preference for sucrose in an acute test, compared to v-WT mice, under both control and restraint conditions (genotype effect F1,20=11.2, P<0.01; stress effect F1,20=3.1, P=0.09; genotype × stress interaction F1,20=0.2, P=0.7; n=4-8/group). f) Sucrose preference remained lower in v-TK mice compared to v-WT mice during the subsequent dark cycle (genotype effect F1,25=6.8, P=0.01; stress effect F1,25=0.8, P=0.4; genotype × stress interaction F1,25=0.5, P=0.5; n=4-10/group). Error bars show s.e.m.
Comment in
- Psychiatric disorders: down with(out) neurogenesis.
Welberg L. Welberg L. Nat Rev Neurosci. 2011 Aug 19;12(9):492-3. doi: 10.1038/nrn3097. Nat Rev Neurosci. 2011. PMID: 21852798 No abstract available. - New neurons maintain efficient stress recovery.
Opendak M, Gould E. Opendak M, et al. Cell Stem Cell. 2011 Oct 4;9(4):287-8. doi: 10.1016/j.stem.2011.09.003. Cell Stem Cell. 2011. PMID: 21982225
Similar articles
- PINK1 deficiency is associated with increased deficits of adult hippocampal neurogenesis and lowers the threshold for stress-induced depression in mice.
Agnihotri SK, Sun L, Yee BK, Shen R, Akundi RS, Zhi L, Duncan MJ, Cass WA, Büeler H. Agnihotri SK, et al. Behav Brain Res. 2019 May 2;363:161-172. doi: 10.1016/j.bbr.2019.02.006. Epub 2019 Feb 5. Behav Brain Res. 2019. PMID: 30735759 - Chronic activation of NPFFR2 stimulates the stress-related depressive behaviors through HPA axis modulation.
Lin YT, Liu TY, Yang CY, Yu YL, Chen TC, Day YJ, Chang CC, Huang GJ, Chen JC. Lin YT, et al. Psychoneuroendocrinology. 2016 Sep;71:73-85. doi: 10.1016/j.psyneuen.2016.05.014. Epub 2016 May 18. Psychoneuroendocrinology. 2016. PMID: 27243477 - Antidepressants recruit new neurons to improve stress response regulation.
Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, Palme R, Griebel G, Ibarguen-Vargas Y, Hen R, Belzung C. Surget A, et al. Mol Psychiatry. 2011 Dec;16(12):1177-88. doi: 10.1038/mp.2011.48. Epub 2011 May 3. Mol Psychiatry. 2011. PMID: 21537331 Free PMC article. - Is there a role for young hippocampal neurons in adaptation to stress?
Dranovsky A, Leonardo ED. Dranovsky A, et al. Behav Brain Res. 2012 Feb 14;227(2):371-5. doi: 10.1016/j.bbr.2011.05.007. Epub 2011 May 19. Behav Brain Res. 2012. PMID: 21621559 Free PMC article. Review. - Glucocorticoid-mediated mechanisms of hippocampal damage: Contribution of subgranular neurogenesis.
Podgorny OV, Gulyaeva NV. Podgorny OV, et al. J Neurochem. 2021 May;157(3):370-392. doi: 10.1111/jnc.15265. Epub 2020 Dec 30. J Neurochem. 2021. PMID: 33301616 Review.
Cited by
- Regulation of Neurogenesis by Organic Cation Transporters: Potential Therapeutic Implications.
Ishimoto T, Kato Y. Ishimoto T, et al. Handb Exp Pharmacol. 2021;266:281-300. doi: 10.1007/164_2021_445. Handb Exp Pharmacol. 2021. PMID: 33782772 - New neurons retire early.
Schoenfeld TJ, Gould E. Schoenfeld TJ, et al. Nat Neurosci. 2012 Dec;15(12):1611-2. doi: 10.1038/nn.3268. Nat Neurosci. 2012. PMID: 23187692 No abstract available. - In vivo imaging of adult human hippocampal neurogenesis: progress, pitfalls and promise.
Ho NF, Hooker JM, Sahay A, Holt DJ, Roffman JL. Ho NF, et al. Mol Psychiatry. 2013 Apr;18(4):404-16. doi: 10.1038/mp.2013.8. Epub 2013 Feb 26. Mol Psychiatry. 2013. PMID: 23439487 Free PMC article. Review. - Long-term exercise training down-regulates m6A RNA demethylase FTO expression in the hippocampus and hypothalamus: an effective intervention for epigenetic modification.
Liu SJ, Cai TH, Fang CL, Lin SZ, Yang WQ, Wei Y, Zhou F, Liu L, Luo Y, Guo ZY, Zhao G, Li YP, Li LM. Liu SJ, et al. BMC Neurosci. 2022 Sep 26;23(1):54. doi: 10.1186/s12868-022-00742-8. BMC Neurosci. 2022. PMID: 36163017 Free PMC article. - Lasting Adaptations in Social Behavior Produced by Social Disruption and Inhibition of Adult Neurogenesis.
Opendak M, Offit L, Monari P, Schoenfeld TJ, Sonti AN, Cameron HA, Gould E. Opendak M, et al. J Neurosci. 2016 Jun 29;36(26):7027-38. doi: 10.1523/JNEUROSCI.4435-15.2016. J Neurosci. 2016. PMID: 27358459 Free PMC article.
References
- Holsboer F, Ising M. Stress hormone regulation: biological role and translation into therapy. Annual review of psychology. 2010;61:81–109. C101–111. - PubMed
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. - PubMed
- Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–238. - PubMed
- Sapolsky RM. Is impaired neurogenesis relevant to the affective symptoms of depression? Biol Psychiatry. 2004;56:137–139. - PubMed
- Drew MR, Hen R. Adult hippocampal neurogenesis as target for the treatment of depression. CNS & neurological disorders drug targets. 2007;6:205–218. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases