Tankyrase-1 function at telomeres and during mitosis is regulated by Polo-like kinase-1-mediated phosphorylation - PubMed (original) (raw)
Tankyrase-1 function at telomeres and during mitosis is regulated by Polo-like kinase-1-mediated phosphorylation
G-H Ha et al. Cell Death Differ. 2012 Feb.
Abstract
Telomere length is critical for chromosome stability that affects cell proliferation and survival. Telomere elongation by telomerase is inhibited by the telomeric protein, TRF1. Tankyrase-1 (TNKS1) poly(ADP-ribosyl)ates TRF1 and releases TRF1 from telomeres, thereby allowing access of telomerase to the telomeres. TNKS1-mediated poly(ADP-ribosyl)ation also appears to be crucial for regulating the mitotic cell cycle. In searching for proteins that interact with polo-like kinase-1 (Plk1) by using complex proteomics, we identified TNKS1 as a novel Plk1-binding protein. Here, we report that Plk1 forms a complex with TNKS1 in vitro and in vivo, and phosphorylates TNKS1. Phosphorylation of TNKS1 by Plk1 appears to increase TNKS1 stability and telomeric poly(ADP-ribose) polymerase (PARP) activity. By contrast, targeted inhibition of Plk1 or mutation of phosphorylation sites decreased the stability and PARP activity of TNKS1, leading to distort mitotic spindle-pole assembly and telomeric ends. Taken together, our results provide evidence of a novel molecular mechanism in which phosphorylation of TNKS1 by Plk1 may help regulate mitotic spindle assembly and promote telomeric chromatin maintenance.
Figures
Figure 1
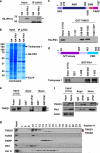
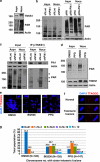
Plk1 interacts with TNKS1 in vitro and in vivo. (a) Lysates from control AGS (a gastric cancer cell line) cells and ones stably overexpressing HA-tagged Plk1 (AGS-HA-Plk1) were prepared for immunoprecipitation using an anti-HA antibody and subjected to immunoblot analysis. The arrowhead and the asterisk indicate HA-PlK1 and IgG heavy chains, respectively. (b) Lysates from the AGS-HA-Plk1 cells were used for immunoprecipitaion using an anti-HA antibody. The eluted HA-Plk1 immune complexes were analyzed by SDS-PAGE and subsequent Coomassie staining followed by mass spectrometric analysis. TNKS1, HA-Plk1, and TCTP (as a positive control) polypeptides are indicated by arrows. (c) Schematic diagrams of TNKS1. HPS, homopolymeric tracts of histidine, proline, and serine repeats; ANK, ankyrin domain; SAM, sterile alpha motif; PARP, poly(ADP-ribose) polymerase. Purified His-tagged Plk1 were incubated with bead-bound GST or GST-TNKS1 (GST-TNKS1) fusion proteins. The eluted GST and GST fusion proteins were resolved by SDS-PAGE prior to immunoblot analyses. (d) Schematic diagrams of Plk1. GST and GST-Plk1 fusion proteins were incubated with HeLa cell lysates cultured in the presence of nocodazole for 12 h. Bead-bound GST or GST-Plk1s were washed, resolved by SDS-PAGE, and immunoblotted using anti-TNKS1 antibody. (e) HeLa cells were cultured in the absence (Asyn) or presence (Noco) of nocodazole. Lysates were incubated with purified GST or GST-Plk1 fusion protein. Bead-bound GST or GST-Plk1s were washed, resolved by SDS-PAGE, and immunoblotted using anti-TNKS1 antibody. (f) HeLa cells were cultured in the absence (Asyn) or presence (Noco) of nocodazole. Lysates were immunoprecipitaed using normal IgG (negative control) or anti-Plk1 antibody. The immunocomplexes were resolved by SDS-PAGE and immunoblotted using and-Plk1, anti-TNKS1, and anti-actin antibodies. (g) Extracts were prepared from HeLa cells synchronized at mitosis by nocodazole, and fractionated by gel filtration using a Superdex-200 column. Fractions (numbered 7–24) were analyzed by immunoblotting using antibodies against TNKS1 (TNKS1), NuMA, PARP, Plk1, and Aurora-A (Aur-A). Input indicates 4% of the extract
Figure 2
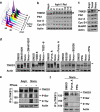
Plk1 and its priming kinase, Cdk1, are capable of phosphorylating TNKS1. (a and b) HeLa cells were treated with aphidicolin (Aphi; 1.6 _μ_g/ml) for 14 h and then released (Aphi/Rel). Cells were harvested at the indicated time points, stained with propidium iodide (PI), and analyzed by flow cytometry to determine their DNA content. (a) Asyn, Asynchronized cells. (b) Lysates from asynchronized or synchronized HeLa cells were analyzed by immunoblotting using anti-TNKS1 (TNKS1), anti-Plk1, anti-Aurora-A (Aur-A), anti-cyclin-B1 (Cyc-B1), anti-BubR1, anti-Bub3, or anti-actin antibodies. (c) HeLa cells were treated with paclitaxel (Taxol, 33 nM) or nocodazole (Noco, 200 ng/ml) for 16 h. Lysates from the asynchronized or synchronized HeLa cells were analyzed by immunoblotting using the indicated antibodies. Asyn, Asynchronized cells. (d) HeLa cells were treated with the various cell-cycle kinase inhibitors for 12 h as indicated. Lysates were immunoblotted using anti-TNKS1 (TNKS1) and anti-actin antibodies. (e) HeLa cells were treated with (Noco) or without (Asyn) nocodazole (100 ng/ml) for 16 h. Cellular extracts were incubated with or without or lambda phosphatase (PPase) and immunoprecipitated using anti-TNKS1 antibody. The eluted TNKS1 protein was resolved by SDS-PAGE and detected using antibodies against TNKS1, phospho-Ser (P-Ser), phospho-Thr (P-Thr), and actin. (f) HeLa cells were treated with nocodazole and then with purvalanol-A (25 _μ_M) or purpurogallin (PPG, 50 _μ_M). Lysates were immunoprecipitated using an anti-TNKS1 antibody, and the resulting TNKS1 protein was probed using anti-phospho-Ser (P-Ser), anti-phospho-Thr (P-Thr), anti-TNKS1 (TNKS1), and anti-actin antibodies
Figure 3
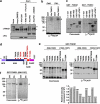
Plk1 directly phosphorylates TNKS1 in vitro and in vivo. (a) Lysates from asynchronized HeLa cell were incubated with 0.2 mM cold ATP either alone (lane-2) or in combination with purified Cdk1 (1 _μ_g), Cdk1 plus purvalanol-A (25 _μ_M), Plk1 (1 _μ_g), or Plk1 (1 _μ_g) plus BI2536 (300 nM) at 37 °C for 1 h. Samples were separated on 8% SDS-PAGE gel and subsequently immunoblotted using anti-TNKS1 and anti-His antibodies. As control, TNKS1 hyper-phosphorylated was observed in nocodazole-treated (Noco) HeLa cells as indicated by an arrowhead and asterisk. (b) Purified His-TNKS1 was incubated with either recombinant Cdk1 and cyclin-B1 (Cdk1) or Plk1 in the presence of [_γ_32P]ATP. Protein samples were resolved by SDS-PAGE and visualized by autoradiography. (c) Purified GST-fused TNKS1 mutant proteins (amino acids 1–157, 158–595, 596–1022, and 1023–1327) or the GST-TRF1 fusion protein (as a positive control) were incubated with recombinant His-Plk1 protein in the presence of [_γ_32P]ATP. GST fusion proteins were resolved by SDS-PAGE and visualized by Coomassie blue staining (left panel) or autoradiography (right panel). (d) Schematic diagrams of TNKS1, including HPS, ANK, SAM, and PARP motifs. Eight putative sites phosphorylated by Plk1 are indicated. (e) Eight putative phosphorylation sites, T643, T785, T839, T930, T938, S978/T982, or T1128, were mutated to alanine (A) using plasmids encoding GST-TNKS1 (596–1022) and GST-TNKS1 (1023–1327). Purified GST-TNKS1 proteins were incubated with recombinant Plk1 in the presence of [_γ_32P]ATP. Protein samples were resolved by SDS-PAGE and visualized by autoradiography. (f) All five putative phosphorylation sites, T839, T930, S978/T982, and T1128, were mutated in GST-TNKS1 (596–1327) to generate GST-TNKS1 (596–1327)-5A. Purified WT GST-TNKS1 (596–1327) and GST-TNKS1 (596–1327)-5A mutant proteins were incubated with recombinant Plk1 in the presence of [_γ_32P] ATP and visualized by autoradiography
Figure 4
Plk1 contributes to the regulation of TNKS1 stability. (a) HeLa cells were transfected with shRNA specifically targeting luciferase (shLuc) or Plk1 (shPlk1). Forty-eight hours after transfection, the cells were cultured in the absence (Asyn) or presence (Noco) of nocodazole for another 16 h. Lysates were prepared and analyzed by immunoblotting using the indicated antibodies. (b) HeLa cells were transfected with shLuc or shPlk1, treated with nocodazole, and then released in normal culture media containing the proteasome inhibitor MG132 (10 μ_m/ml) or DMSO. Cell lysates were analyzed by immunoblotting using anti-TNKS1, anti-BubR1 (a positive control), anti-Plk1, anti-GSK3_β (a negative control), and anti-actin antibodies. (c) HeLa cells were treated with the proteasome inhibitor, MG132 (10 _μ_m/ml), for 6 h, and then harvested for immunoblot analysis using antibodies against TNKS1, Plk1, NuMA, TRF1, and actin. (d) HeLa cells were transfected with shLuc or two different shPlk1s, shPlk1 #1 (targeting Plk1 ORF) and shPlk1 #2, (targeting Plk1 3′-UTR), as described under Materials and Methods. Thirty-six hours after transfection, lysates were prepared and subjected to immunoblot analysis using anti-TNKS1, anti-Plk1, anti-TRF1, anti-actin antibodies. (e) HeLa cells were co-transfected with shRNA specific for Plk1 3′-UTR (shPlk1 #2) and expression plasmids encoding shRNA-insensitive (shi), HA-tagged WT Plk1 (HA-Plk1 WT) or a kinase-dead mutant of Plk1 (HA-Plk1 K82R). Thirty-six hours after transfection, lysates were prepared and subjected to immunoblot analysis using the indicated antibodies. (f) U2OS cells were immunostained using an anti-Plk1 (green) or an anti-TNKS1 (TNKS1, red) antibody, or CREST serum (purple). The cells were examined by confocal microscopy. (g) HeLa cells were transfected with shLuc (a negative control) or shPlk1. Thirty-six hours after transfection, the cells were fixed with 4% paraformaldehyde and immunostained using anti-Plk1 (green) or anti-TNKS1 (red) antibodies
Figure 5
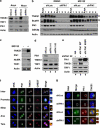
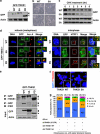
Poly(ADP-ribosyl)ation activity of TNKS1 is regulated by Plk1-mediated hyper-phosphorylation. (a) HeLa cell lysates were prepared in a nuclear extraction buffer from asynchronous (Asyn) or nocodazole-treated (Noco, 200 ng/ml) cells. Lysates were prepared and analyzed by immunoblotting using anti-TNKS1, anti-PAR, or anti-actin antibodies (as a loading control). (b) HeLa cells were transfected with shRNA (shLuc or shPlk1; left panel), pHA, or pHA-Plk1 (right panel). Forty-eight hours after transfection, the cells were cultured in the absence (Asyn) or presence (Noco) of nocodazole for another 16 h. Lysates were separated by electrophoresis and immunoblotted using anti-PAR and anti-actin antibodies. (c) HeLa cells were transfected with shLuc or shPlk1, and cultured in the absence (Asyn) or presence (Noco) of nocodazole for an additional 16 h. Lysates (4 mg of total protein) were used for immunoprecipitation (IP) using anti-TNKS1 antibody (áTNKS1) and analyzed by immunoblotting using anti-Plk1, anti-actin, anti-PAR, or anti-TNKS1 antibodies. (d) Asynchronous (Asyn) or nocodazole-treated (Noco) HeLa cells were further incubated in the absence (DMSO) or presence of a Plk1 inhibitor (50 _μ_M PPG). Lysates were prepared, resolved by SDS-PAGE, and analyzed by immunoblotting using anti-PAR and anti-actin antibodies. (e) Telomeric PNA FISH analysis (FISH using peptide nucleic acid probes) was performed using metaphase spreads of H1299 cells treated with BI2536 (60 nM) or PPG (50 _μ_M) for 12 h, swollen in hypotonic buffer, and fixed in methanol-acetic acid solution. Telomeric repeats were detected by using a Cy3-(CCCTAA)3 PNA probe (red) and DNA was stained with DAPI (blue). (f) Representative images of normal sister telomeres and telomeric fusion in the metaphase spreads of Plk1 inhibitor-treated H1299 cells. Note that in some cases sister telomeric fusions appeared at both ends of the chromosome. (g) The chromosome number distribution of sister telomeric fusions observed in each metaphase was determined and plotted in a graph. More than 100 cells were scored by FISH
Figure 6
Mutations of TNKS1 Plk1-mediated phosphorylation sites reduce TNKS1 stability and alter spindle-pole localization and telomere separation. (a) Plasmids encoding GFP-fused full-length TNKS1 WT (GFP-TNKS1 WT), TNKS1-5A, or TNKS1 PD were transfected into HeLa cells. Proteins in the lysates from the cells were separated by electrophoresis and immunoblotted using anti-GFP and actin antibodies. (b) HeLa cells transfected with plasmids encoding GFP-TNKS1 WT or GFP-TNKS1-5A were observed by phase-contrast microscopy 60 h after transfection. (c) Asynchronized HeLa cells were transfected with plasmids encoding GFP-TNKS1 WT or GFP-TNKS1-5A and then treated with cycloheximide (CHX). Lysates were analyzed by immunoblotting using anti-GFP and anti-actin antibodies. (d) Transfected HeLa cells described in panel a were immunostained using anti-Aurora-A (red, left panels) or anti-hTRF1 (red, right panels) antibodies and DNA was visualized by DAPI staining. The cells were examined by confocal microscopy. (e) HeLa cells were transfected with GFP-TNKS1 WT, GFP-TNKS1-5A or GFP-TNKS1 PD, and treated then with nocodazole. Cellular extracts were immunoprecipitated using anti-GFP antibody. The eluted GFP-TNKS1 proteins were resolved by SDS-PAGE and probed using antibodies against TNKS1, PAR, and actin. (f and g) HeLa cells were co-transfected with shRNA specifically targeting TNKS1 3′-UTR and expression plasmids encoding shRNA-insensitive (shi), WT GFP-TNKS1 (shi TNKS1 WT) or GFP-TNKS1-5A (shi TNKS1 5A). Telomeric DNA FISH analysis (FISH using peptide nucleic acid probes) was performed on metaphase spreads of transfected HeLa cells swollen in hypotonic buffer and fixed in a methanol-acetic acid solution. Telomeric repeats were detected by using a Cy3-(CCCTAA)3 PNA probe (red) and DNA was stained with DAPI (blue). (f) Representative images of normal sister telomeres (in cells expressing TNKS1 WT) and telomeric fusions (in cells expressing TNKS1-5A mutant) of metaphase spreads. Note that in some cases sister telomeric fusions appeared at both ends of the chromosomes. (g) The chromosome number distribution of the sister telomeric fusions observed in each metaphase was calculated and plotted on a graph. More than 100 cells from each transfectant cell line were scored by FISH
Similar articles
- Tankyrase1-mediated poly(ADP-ribosyl)ation of TRF1 maintains cell survival after telomeric DNA damage.
Yang L, Sun L, Teng Y, Chen H, Gao Y, Levine AS, Nakajima S, Lan L. Yang L, et al. Nucleic Acids Res. 2017 Apr 20;45(7):3906-3921. doi: 10.1093/nar/gkx083. Nucleic Acids Res. 2017. PMID: 28160604 Free PMC article. - Plk1 phosphorylation of TRF1 is essential for its binding to telomeres.
Wu ZQ, Yang X, Weber G, Liu X. Wu ZQ, et al. J Biol Chem. 2008 Sep 12;283(37):25503-25513. doi: 10.1074/jbc.M803304200. Epub 2008 Jul 14. J Biol Chem. 2008. PMID: 18625707 Free PMC article. - Telomere elongation by a mutant tankyrase 1 without TRF1 poly(ADP-ribosyl)ation.
Muramatsu Y, Tahara H, Ono T, Tsuruo T, Seimiya H. Muramatsu Y, et al. Exp Cell Res. 2008 Mar 10;314(5):1115-24. doi: 10.1016/j.yexcr.2007.12.005. Epub 2007 Dec 14. Exp Cell Res. 2008. PMID: 18221737 - Playing polo during mitosis: PLK1 takes the lead.
Combes G, Alharbi I, Braga LG, Elowe S. Combes G, et al. Oncogene. 2017 Aug 24;36(34):4819-4827. doi: 10.1038/onc.2017.113. Epub 2017 Apr 24. Oncogene. 2017. PMID: 28436952 Review. - Polo-like kinase 1: target and regulator of anaphase-promoting complex/cyclosome-dependent proteolysis.
Eckerdt F, Strebhardt K. Eckerdt F, et al. Cancer Res. 2006 Jul 15;66(14):6895-8. doi: 10.1158/0008-5472.CAN-06-0358. Cancer Res. 2006. PMID: 16849530 Review.
Cited by
- Tankyrase inhibition preserves osteoarthritic cartilage by coordinating cartilage matrix anabolism via effects on SOX9 PARylation.
Kim S, Han S, Kim Y, Kim HS, Gu YR, Kang D, Cho Y, Kim H, Lee J, Seo Y, Chang MJ, Chang CB, Kang SB, Kim JH. Kim S, et al. Nat Commun. 2019 Oct 25;10(1):4898. doi: 10.1038/s41467-019-12910-2. Nat Commun. 2019. PMID: 31653858 Free PMC article. - Causes of genome instability: the effect of low dose chemical exposures in modern society.
Langie SA, Koppen G, Desaulniers D, Al-Mulla F, Al-Temaimi R, Amedei A, Azqueta A, Bisson WH, Brown DG, Brunborg G, Charles AK, Chen T, Colacci A, Darroudi F, Forte S, Gonzalez L, Hamid RA, Knudsen LE, Leyns L, Lopez de Cerain Salsamendi A, Memeo L, Mondello C, Mothersill C, Olsen AK, Pavanello S, Raju J, Rojas E, Roy R, Ryan EP, Ostrosky-Wegman P, Salem HK, Scovassi AI, Singh N, Vaccari M, Van Schooten FJ, Valverde M, Woodrick J, Zhang L, van Larebeke N, Kirsch-Volders M, Collins AR. Langie SA, et al. Carcinogenesis. 2015 Jun;36 Suppl 1(Suppl 1):S61-88. doi: 10.1093/carcin/bgv031. Carcinogenesis. 2015. PMID: 26106144 Free PMC article. Review. - Wnt/beta-catenin signaling and small molecule inhibitors.
Voronkov A, Krauss S. Voronkov A, et al. Curr Pharm Des. 2013;19(4):634-64. doi: 10.2174/138161213804581837. Curr Pharm Des. 2013. PMID: 23016862 Free PMC article. Review. - Tankyrase: a promising therapeutic target with pleiotropic action.
Sagathia V, Patel C, Beladiya J, Patel S, Sheth D, Shah G. Sagathia V, et al. Naunyn Schmiedebergs Arch Pharmacol. 2023 Dec;396(12):3363-3374. doi: 10.1007/s00210-023-02576-5. Epub 2023 Jun 20. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 37338576 Review. - Interaction of tankyrase and peroxiredoxin II is indispensable for the survival of colorectal cancer cells.
Kang DH, Lee DJ, Lee S, Lee SY, Jun Y, Kim Y, Kim Y, Lee JS, Lee DK, Lee S, Jho EH, Yu DY, Kang SW. Kang DH, et al. Nat Commun. 2017 Jun 28;8(1):40. doi: 10.1038/s41467-017-00054-0. Nat Commun. 2017. PMID: 28659575 Free PMC article.
References
- Petronczki M, Lenart P, Peters JM. Polo on the rise-from mitotic entry to cytokinesis with Plk1. Dev Cell. 2008;14:646–659. - PubMed
- Takai N, Hamanaka R, Yoshimatsu J, Miyakawa I. Polo-like kinases (Plks) and cancer. Oncogene. 2005;24:287–291. - PubMed
- Simizu S, Osada H. Mutations in the Plk gene lead to instability of Plk protein in human tumour cell lines. Nat Cell Biol. 2000;2:852–854. - PubMed
- Lapenna S, Giordano A. Cell cycle kinases as therapeutic targets for cancer. Nat Rev Drug Discov. 2009;8:547–566. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous