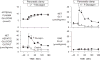Physiologic action of glucagon on liver glucose metabolism - PubMed (original) (raw)
Review
Physiologic action of glucagon on liver glucose metabolism
C J Ramnanan et al. Diabetes Obes Metab. 2011 Oct.
Abstract
Glucagon is a primary regulator of hepatic glucose production (HGP) in vivo during fasting, exercise and hypoglycaemia. Glucagon also plays a role in limiting hepatic glucose uptake and producing the hyperglycaemic phenotype associated with insulin deficiency and insulin resistance. In response to a physiological rise in glucagon, HGP is rapidly stimulated. This increase in HGP is entirely attributable to an enhancement of glycogenolysis, with little to no acute effect on gluconeogenesis. This dramatic rise in glycogenolysis in response to hyperglucagonemia wanes with time. A component of this waning effect is known to be independent of hyperglycemia, though the molecular basis for this tachyphylaxis is not fully understood. In the overnight fasted state, the presence of basal glucagon secretion is essential in countering the suppressive effects of basal insulin, resulting in the maintenance of appropriate levels of glycogenolysis, fasting HGP and blood glucose. The enhancement of glycogenolysis in response to elevated glucagon is critical in the life-preserving counterregulatory response to hypoglycaemia, as well as a key factor in providing adequate circulating glucose for working muscle during exercise. Finally, glucagon has a key role in promoting the catabolic consequences associated with states of deficient insulin action, which supports the therapeutic potential in developing glucagon receptor antagonists or inhibitors of glucagon secretion.
© 2011 Blackwell Publishing Ltd.
Conflict of interest statement
Conflict of Interests
The authors declare no conflicts of interests.
Figures
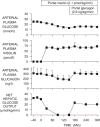
Figure 1
The effect of a selective rise in plasma glucagon brought about in conscious overnight fasted dogs maintained with a pancreatic clamp (somatostatin infusion, basal portal vein insulin replacement). This group was compared to control animals (somatostatin infusion, basal portal vein insulin and glucagon infusion) that were infused peripherally with glucose to match the glycemic level of the ‘high glucagon’ group. Net GLY = net hepatic glycogenolytic flux; GNG flux = gluconeogenic flux. Filled symbols refer to the response to a rise in glucagon infusion. Open symbols refer to the hyperglycaemic control studies. Adapted with permission from Ref. [1].
Figure 2
The effect of a selective fall in plasma glucagon brought about in conscious overnight fasted dogs maintained with a pancreatic clamp (somatostatin infusion, basal portal vein insulin infusion). The control group (open symbols) was maintained on basal infusion of insulin and glucagon intraportally. In the test group (filled symbols), glucagon infusion was stopped at 0 min and peripheral glucose infusion was required to maintain euglycemia. Net GLY = net hepatic glycogenolytic flux; GNG flux = gluconeogenic flux. Adapted with permission from Ref. [1].
Figure 3
Dose response relationship between the hepatic sinusoidal glucagon level and the initial (15 min) increase in glucose production in humans and dogs with insulin clamped at a basal value. Hepatic sinusoidal glucagon concentrations in the dog were calculated from measured arterial and portal vein glucagon concentrations and the measured rates of blood flow in these vessels. In humans the concentrations were extrapolated from arterial plasma glucagon levels. Adapted with permission from Ref. [1].
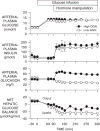
Figure 4
The impact of plasma glucagon on net hepatic glucose uptake under hyperglycemic-hyperinsulinemic conditions in conscious 42 h fasted dogs. At 0 min, a pancreatic clamp was initiated (infusion of somatostatin and basal amounts of insulin and glucagon into the portal vein). Hyperglycemia was brought about using peripheral vein glucose infusion beginning at 0 min. At 90 min (hormone manipulation period), the portal vein infusion of insulin was increased (~fourfold) and glucose was infused intraportally (22 μmol/kg/min) to mimic a meal response. At the same time, the portal vein glucagon infusion was altered to create either an elevated or low glucagon level. Adapted with permission from Ref. [16].
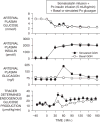
Figure 5
The role of glucagon in defense of a low blood sugar. Pancreatic clamps (somatostatin + portal vein insulin infusion at a 20-fold basal rate) were used in two groups of conscious overnight fasted dogs. In one group the normal glucagon response to hypoglycemia was reproduced using a variable portal vein glucagon infusion. In the other glucagon was kept basal. Adapted with permission from Ref. [22].
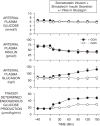
Figure 6
The role of glucagon in support of an exercise-induced increase in glucose utilization. Pancreatic clamps were used in two groups of conscious overnight fasted dogs (somatostatin infusion + replacement of insulin and glucagon via the portal vein). After a basal period (−40 to 0 min), treadmill exercise was initiated and the insulin infusion was decreased (to mimic the insulin level that would normally occur during exercise). Glucagon infusion was either increased (to mimic the glucagon level that would normally occur in response to exercise) or kept basal. Adapted with permission from Ref. [24].
Figure 7
The impact of normalizing plasma insulin and glucagon in pancreatectomized dogs withdrawn from insulin and fasted overnight. Adapted with permission from Ref. [29].
Similar articles
- Integration of metabolic flux with hepatic glucagon signaling and gene expression profiles in the conscious dog.
Coate KC, Ramnanan CJ, Smith M, Winnick JJ, Kraft G, Irimia-Dominguez J, Farmer B, Donahue EP, Roach PJ, Cherrington AD, Edgerton DS. Coate KC, et al. Am J Physiol Endocrinol Metab. 2024 Apr 1;326(4):E428-E442. doi: 10.1152/ajpendo.00316.2023. Epub 2024 Feb 7. Am J Physiol Endocrinol Metab. 2024. PMID: 38324258 - A physiologic increase in brain glucagon action alters the hepatic gluconeogenic/glycogenolytic ratio but not glucagon's overall effect on glucose production.
Edgerton DS, Kraft G, Smith M, Farmer B, Williams P, Cherrington AD. Edgerton DS, et al. Am J Physiol Endocrinol Metab. 2023 Feb 1;324(2):E199-E208. doi: 10.1152/ajpendo.00304.2022. Epub 2023 Jan 18. Am J Physiol Endocrinol Metab. 2023. PMID: 36652399 Free PMC article. - Splanchnic regulation of glucose production.
Wahren J, Ekberg K. Wahren J, et al. Annu Rev Nutr. 2007;27:329-45. doi: 10.1146/annurev.nutr.27.061406.093806. Annu Rev Nutr. 2007. PMID: 17465853 Review. - Glucose metabolism during exercise in man: the role of insulin and glucagon in the regulation of hepatic glucose production and gluconeogenesis.
Lavoie C, Ducros F, Bourque J, Langelier H, Chiasson JL. Lavoie C, et al. Can J Physiol Pharmacol. 1997 Jan;75(1):26-35. doi: 10.1139/cjpp-75-1-26. Can J Physiol Pharmacol. 1997. PMID: 9101062 - Control of hepatic glucose output by glucagon and insulin in the intact dog.
Cherrington AD, Chiasson JL, Liljenquist JE, Lacy WW, Park CR. Cherrington AD, et al. Biochem Soc Symp. 1978;(43):31-45. Biochem Soc Symp. 1978. PMID: 373768 Review.
Cited by
- Directed differentiation of pancreatic δ cells from human pluripotent stem cells.
Chen L, Wang N, Zhang T, Zhang F, Zhang W, Meng H, Chen J, Liao Z, Xu X, Ma Z, Xu T, Liu H. Chen L, et al. Nat Commun. 2024 Jul 27;15(1):6344. doi: 10.1038/s41467-024-50611-7. Nat Commun. 2024. PMID: 39068220 Free PMC article. - Spatial metabolomics and its application in the liver.
Santos AA, Delgado TC, Marques V, Ramirez-Moncayo C, Alonso C, Vidal-Puig A, Hall Z, Martínez-Chantar ML, Rodrigues CMP. Santos AA, et al. Hepatology. 2024 May 1;79(5):1158-1179. doi: 10.1097/HEP.0000000000000341. Epub 2023 Feb 23. Hepatology. 2024. PMID: 36811413 Free PMC article. Review. - Cancer-Induced Reprogramming of Host Glucose Metabolism: "Vicious Cycle" Supporting Cancer Progression.
Schwartsburd P. Schwartsburd P. Front Oncol. 2019 Apr 4;9:218. doi: 10.3389/fonc.2019.00218. eCollection 2019. Front Oncol. 2019. PMID: 31019893 Free PMC article. - Evaluating the effectiveness of a novel somatostatin receptor 2 antagonist, ZT-01, for hypoglycemia prevention in a rodent model of type 2 diabetes.
D'Souza NC, Aiken JA, Hoffman EG, Atherley SC, Champsi S, Aleali N, Shakeri D, El-Zahed M, Akbarian N, Nejad-Mansouri M, Bavani PZ, Liggins RL, Chan O, Riddell MC. D'Souza NC, et al. Front Pharmacol. 2024 Feb 28;15:1302015. doi: 10.3389/fphar.2024.1302015. eCollection 2024. Front Pharmacol. 2024. PMID: 38510652 Free PMC article. - Integration of metabolic flux with hepatic glucagon signaling and gene expression profiles in the conscious dog.
Coate KC, Ramnanan CJ, Smith M, Winnick JJ, Kraft G, Irimia-Dominguez J, Farmer B, Donahue EP, Roach PJ, Cherrington AD, Edgerton DS. Coate KC, et al. Am J Physiol Endocrinol Metab. 2024 Apr 1;326(4):E428-E442. doi: 10.1152/ajpendo.00316.2023. Epub 2024 Feb 7. Am J Physiol Endocrinol Metab. 2024. PMID: 38324258
References
- Cherrington AD. Control of glucose uptake and release by the liver in vivo. Banting Lecture 1997. Diabetes. 1999;48:1198–1214. - PubMed
- Wada M, Connolly CC, Tarumi C, Neal DW, Cherrington AD. Hepatic denervation does not change the response of the liver to glucagon in the conscious dog. Am J Physiol. 1995;268:E194–E203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical