Evidence for ordering of Alzheimer disease biomarkers - PubMed (original) (raw)
Comparative Study
Evidence for ordering of Alzheimer disease biomarkers
Clifford R Jack Jr et al. Arch Neurol. 2011 Dec.
Abstract
Objective: To empirically assess the concept that Alzheimer disease (AD) biomarkers significantly depart from normality in a temporally ordered manner.
Design: Validation sample.
Setting: Multisite, referral centers.
Participants: A total of 401 elderly participants in the Alzheimer's Disease Neuroimaging Initiative who were cognitively normal, who had mild cognitive impairment, or who had AD dementia. We compared the proportions of 3 AD biomarker values (the Aβ42 level in cerebrospinal fluid [CSF], the total tau level in CSF, and the hippocampal volume adjusted for intracranial volume [hereafter referred to as the adjusted hippocampal volume]) that were abnormal as cognitive impairment worsened. Cut points demarcating normal vs abnormal for each biomarker were established by maximizing diagnostic accuracy in independent autopsy samples.
Main outcome measures: Three AD biomarkers (ie, the CSF Aβ42 level, the CSF total tau level, and the adjusted hippocampal volume).
Results: Within each clinical group of the entire sample (n = 401), the CSF Aβ42 level was abnormal more often than was the CSF total tau level or the adjusted hippocampal volume. Among the 298 participants with both baseline and 12-month data, the proportion of participants with an abnormal Aβ42 level did not change from baseline to 12 months in any group. The proportion of participants with an abnormal total tau level increased from baseline to 12 months in cognitively normal participants (P = .05) but not in participants with mild cognitive impairment or AD dementia. For 209 participants with an abnormal CSF Aβ42 level at baseline, the percentage with an abnormal adjusted hippocampal volume but normal CSF total tau level increased from baseline to 12 months in participants with mild cognitive impairment. No change in the percentage of MCI participants with an abnormal total tau level was seen between baseline and 12 months.
Conclusions: A reduction in the CSF Aβ42 level denotes a pathophysiological process that significantly departs from normality (ie, becomes dynamic) early, whereas the CSF total tau level and the adjusted hippocampal volume are biomarkers of downstream pathophysiological processes. The CSF total tau level becomes dynamic before the adjusted hippocampal volume, but the hippocampal volume is more dynamic in the clinically symptomatic mild cognitive impairment and AD dementia phases of the disease than is the CSF total tau level.
Figures
Figure 1
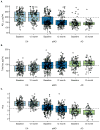
Box plots and superimposed data points showing the distribution of biomarkers by baseline diagnosis and visit. Boxes indicate the quartiles and have whiskers extending to the furthest data point within 1.5 inter-quartile ranges of the box. T-tau is shown on the log scale. In subjects with both baseline and 12 month data, CSF Aβ42 is not changing from baseline to 12 months (CN, p=0.52; MCI, p=0.13; AD, p=0.51). T-tau is increasing from baseline to 12 months in CN subjects (p=0.002) but not in MCI or AD (p=0.12, p=0.36). HVa is decreasing in all clinical groups (p<0.001).
Figure 2
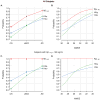
Estimated percentages of abnormality for each biomarker in all subjects (n=401) and within the subset of subjects with abnormal baseline CSF Aβ (n=274). Panels A & C show abnormality by clinical diagnosis and panels B & D show abnormality by MMSE score. Cutoffs used are 192 pg/mL for Aβ1–42, 93 for total tau, and 0.48 for HVa.
Figure 3
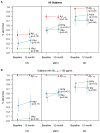
Estimated percentages of abnormality for each biomarker at baseline and 12 months by baseline clinical diagnosis for the subset of subjects with serial data (panel A, n=298) and those with serial data who were CSF Aβ positive at baseline (panel B, n=209). Values are shifted slightly along the x-axis for the middle biomarker to reduce overlap in lines. Cutoffs used are 192 pg/mL for Aβ1–42, 93 for total tau, and 0.48 for HVa.
Comment in
- Ordering of Alzheimer disease biomarkers.
Glodzik L, Galvin J, Pirraglia E, de Leon M. Glodzik L, et al. Arch Neurol. 2012 Mar;69(3):414; author reply 414-5. doi: 10.1001/archneurol.2011.2906. Arch Neurol. 2012. PMID: 22410455 No abstract available.
Similar articles
- ApoE4 effects on automated diagnostic classifiers for mild cognitive impairment and Alzheimer's disease.
Apostolova LG, Hwang KS, Kohannim O, Avila D, Elashoff D, Jack CR Jr, Shaw L, Trojanowski JQ, Weiner MW, Thompson PM; Alzheimer's Disease Neuroimaging Initiative. Apostolova LG, et al. Neuroimage Clin. 2014 Jan 4;4:461-72. doi: 10.1016/j.nicl.2013.12.012. eCollection 2014. Neuroimage Clin. 2014. PMID: 24634832 Free PMC article. - Association Between Longitudinal Plasma Neurofilament Light and Neurodegeneration in Patients With Alzheimer Disease.
Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K. Mattsson N, et al. JAMA Neurol. 2019 Jul 1;76(7):791-799. doi: 10.1001/jamaneurol.2019.0765. JAMA Neurol. 2019. PMID: 31009028 Free PMC article. - Addition of the Aβ42/40 ratio to the cerebrospinal fluid biomarker profile increases the predictive value for underlying Alzheimer's disease dementia in mild cognitive impairment.
Baldeiras I, Santana I, Leitão MJ, Gens H, Pascoal R, Tábuas-Pereira M, Beato-Coelho J, Duro D, Almeida MR, Oliveira CR. Baldeiras I, et al. Alzheimers Res Ther. 2018 Mar 20;10(1):33. doi: 10.1186/s13195-018-0362-2. Alzheimers Res Ther. 2018. PMID: 29558986 Free PMC article. - CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).
Ritchie C, Smailagic N, Noel-Storr AH, Ukoumunne O, Ladds EC, Martin S. Ritchie C, et al. Cochrane Database Syst Rev. 2017 Mar 22;3(3):CD010803. doi: 10.1002/14651858.CD010803.pub2. Cochrane Database Syst Rev. 2017. PMID: 28328043 Free PMC article. Review. - Cerebrospinal fluid in the differential diagnosis of Alzheimer's disease: an update of the literature.
Milos T, Vuic B, Balic N, Farkas V, Nedic Erjavec G, Svob Strac D, Nikolac Perkovic M, Pivac N. Milos T, et al. Expert Rev Neurother. 2024 Nov;24(11):1063-1079. doi: 10.1080/14737175.2024.2400683. Epub 2024 Sep 4. Expert Rev Neurother. 2024. PMID: 39233323 Review.
Cited by
- Three dimensions of the amyloid hypothesis: time, space and 'wingmen'.
Musiek ES, Holtzman DM. Musiek ES, et al. Nat Neurosci. 2015 Jun;18(6):800-6. doi: 10.1038/nn.4018. Nat Neurosci. 2015. PMID: 26007213 Free PMC article. Review. - Impact of the Alzheimer's Disease Neuroimaging Initiative, 2004 to 2014.
Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Cedarbaum J, Donohue MC, Green RC, Harvey D, Jack CR Jr, Jagust W, Morris JC, Petersen RC, Saykin AJ, Shaw L, Thompson PM, Toga AW, Trojanowski JQ; Alzheimer's Disease Neuroimaging Initiative. Weiner MW, et al. Alzheimers Dement. 2015 Jul;11(7):865-84. doi: 10.1016/j.jalz.2015.04.005. Alzheimers Dement. 2015. PMID: 26194320 Free PMC article. Review. - Effect of Alzheimer's disease risk genes on trajectories of cognitive function in the Cardiovascular Health Study.
Sweet RA, Seltman H, Emanuel JE, Lopez OL, Becker JT, Bis JC, Weamer EA, DeMichele-Sweet MA, Kuller LH. Sweet RA, et al. Am J Psychiatry. 2012 Sep;169(9):954-62. doi: 10.1176/appi.ajp.2012.11121815. Am J Psychiatry. 2012. PMID: 22952074 Free PMC article. - Cerebrospinal fluid biomarkers of Alzheimer's disease in healthy elderly.
Randall C, Mosconi L, de Leon M, Glodzik L. Randall C, et al. Front Biosci (Landmark Ed). 2013 Jun 1;18(3):1150-73. doi: 10.2741/4170. Front Biosci (Landmark Ed). 2013. PMID: 23747874 Free PMC article. Review. - Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers.
Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Jack CR Jr, et al. Lancet Neurol. 2013 Feb;12(2):207-16. doi: 10.1016/S1474-4422(12)70291-0. Lancet Neurol. 2013. PMID: 23332364 Free PMC article.
References
- Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 2010 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical