Elevated 17β-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses - PubMed (original) (raw)
Elevated 17β-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses
Dionne P Robinson et al. PLoS Pathog. 2011 Jul.
Abstract
Studies of the 1918 H1N1 influenza pandemic, the H5N1 avian influenza outbreak, and the 2009 H1N1 pandemic illustrate that sex and pregnancy contribute to severe outcome from infection, suggesting a role for sex steroids. To test the hypothesis that the sexes respond differently to influenza, the pathogenesis of influenza A virus infection was investigated in adult male and female C57BL/6 mice. Influenza infection reduced reproductive function in females and resulted in greater body mass loss, hypothermia, and mortality in females than males. Whereas lung virus titers were similar between the sexes, females had higher induction of proinflammatory cytokines and chemokines, including TNF-α, IFN-γ, IL-6, and CCL2, in their lungs than males. Removal of the gonads in both sexes eliminated the sex difference in influenza pathogenesis. Manipulation of testosterone or dihydrotestosterone concentrations in males did not significantly impact virus pathogenesis. Conversely, females administered high doses of estradiol had a ≥10-fold lower induction of TNF-α and CCL2 in the lungs and increased rates of survival as compared with females that had either low or no estradiol. The protective effects of estradiol on proinflammatory cytokines and chemokines, morbidity, and mortality were primarily mediated by signaling through estrogen receptor α (ERα). In summary, females suffer a worse outcome from influenza A virus infection than males, which can be reversed by administration of high doses of estradiol to females and reflects differences in the induction of proinflammatory responses and not in virus load.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
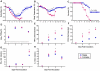
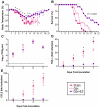
Figure 1. Influenza virus infection causes greater morbidity and mortality in females than males.
Male and female mice were inoculated with PR8 and monitored for changes in body mass (A), rectal temperature (B), and survival (C) for up to 21 days (n = 15/sex). Infectious virus titers (D) as well as concentrations of TNF-α (E), CCL2 (F), IFN-γ (G), and IL-6 (H) were measured in homogenates of lungs removed 0, 1, 3, 5, or 7 days p.i. (n = 10–15/sex/time-point). Fold change represents concentrations of proteins at Days 1–7 p.i. relative to concentrations at Day 0. Data represent the mean ± SEM. The dotted line in panel D represents the limit of detection for the assay. Significant differences between the sexes as determined by post hoc analyses at each time-point are represented by an asterisk (*), P<0.05.
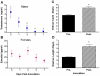
Figure 2. Influenza virus infection alters sex steroid concentrations and reproductive function in males and females.
Male and female mice (n = 10/sex/time-point) were inoculated with PR8, serum was collected, and T concentrations in males (A) and E2 concentrations in females (B) were analyzed Days 0, 1, 3, 5, or 7 p.i. To assess estrous cycles, females (n = 16) were sampled daily by vaginal lavage before and after inoculation with PR8 and the duration of the estrous cycle (C) and diestrus (D) was quantified. For sex steroid hormone measurements, plasma from uninfected animals were collected at the same time as other samples and designated as Day 0 p.i. Data represent the mean ± SEM. Significant differences between Day 0 and other time-points p.i. based on post hoc analyses are represented by an asterisk (*), P<0.05.
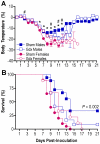
Figure 3. Removal of the gonads reduces the sex difference in influenza pathogenesis.
Rectal temperature (A) and survival (B) were monitored for 21 days after inoculation of intact (sham) male (n = 18), sham female (n = 20), gonadectomized (gdx) male (n = 12), or gdx female (n = 9) mice with PR8. Data represent the mean ± SEM. Significant differences between sham males and females as determined by post hoc analyses at each time-point are represented by an asterisk (*), P<0.05. Significant differences between intact and gdx males as determined by post hoc analyses at each time-point are represented by a number symbol (#), P<0.05.
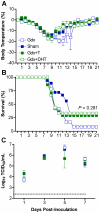
Figure 4. Androgen replacement does not significantly affect morbidity or mortality from influenza virus infection in males.
Males were left intact (sham; n = 10–18/time-point/experiment) or gonadectomized (gdx) and implanted with silastic capsules that were empty (n = 9–12/time-point/experiment) or filled with testosterone (T; n = 8–14/time-point/experiment) or dihydrotestosterone (DHT; n = 10). Males were inoculated with PR8 and monitored daily for changes in body temperature (A) and survival (B). Infectious virus titers (C) were measured by TCID50 in lungs removed Days 1, 3, 5, or 7 p.i. The dotted line in panel C represents the limit of detection for the assay. Data represent the mean ± SEM.
Figure 5. Exogenous E2 protects females against influenza virus infection.
Females were intact (sham; n = 9–20/time-point/experiment), gonadectomized (gdx; n = 7–10/time-point/experiment), or gdx and treated with E2 (n = 8–12/time-point/experiment). Females were inoculated with PR8 and monitored daily for changes in rectal temperature (A) and survival (B). Infectious virus titers (C) as well as concentrations of TNF-α (D) and CCL2 (E) were measured in lungs removed Days 1, 3, 5, or 7 p.i. Fold change represents concentrations of proteins at Days 1–7 p.i. relative to concentrations at Day 0. Data represent the mean ± SEM. The dotted line in panel C represents the limit of detection for the assay. Significant differences between E2-treated and sham or gdx females, as determined by post hoc analyses, are represented by an asterisk (*), P<0.05.
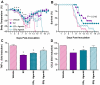
Figure 6. Estradiol protects females against influenza through ERα signaling.
Females were gonadectomized (gdx) and assigned to received vehicle (n = 9–12/time-point/experiment), E2 (n = 9–10/time-point/experiment), the ERα agonist (PPT; n = 9–10/time-point/treatment), or the ERβ agonist (DPN; n = 8–9/time-point/experiment). Females were inoculated with PR8 and monitored daily for changes in rectal temperature (A) and survival (B). Concentrations of TNF-α (C) and CCL2 (D) were quantified in lung homogenates collected from females at Days 0 and 7 p.i. Fold change represents concentrations of proteins at Day 7 p.i. relative to concentrations at Day 0. Data represent the mean ± SEM. Significant differences between treatment groups and vehicle controls, as determined by post hoc analyses, are represented by an asterisk (*), P<0.05.
Similar articles
- 17β-estradiol protects females against influenza by recruiting neutrophils and increasing virus-specific CD8 T cell responses in the lungs.
Robinson DP, Hall OJ, Nilles TL, Bream JH, Klein SL. Robinson DP, et al. J Virol. 2014 May;88(9):4711-20. doi: 10.1128/JVI.02081-13. Epub 2014 Feb 12. J Virol. 2014. PMID: 24522912 Free PMC article. - Production of amphiregulin and recovery from influenza is greater in males than females.
Vermillion MS, Ursin RL, Kuok DIT, Vom Steeg LG, Wohlgemuth N, Hall OJ, Fink AL, Sasse E, Nelson A, Ndeh R, McGrath-Morrow S, Mitzner W, Chan MCW, Pekosz A, Klein SL. Vermillion MS, et al. Biol Sex Differ. 2018 Jul 17;9(1):24. doi: 10.1186/s13293-018-0184-8. Biol Sex Differ. 2018. PMID: 30012205 Free PMC article. - Testosterone Protects Against Severe Influenza by Reducing the Pro-Inflammatory Cytokine Response in the Murine Lung.
Tuku B, Stanelle-Bertram S, Sellau J, Beck S, Bai T, Kouassi NM, Preuß A, Hoenow S, Renné T, Lotter H, Gabriel G. Tuku B, et al. Front Immunol. 2020 Apr 22;11:697. doi: 10.3389/fimmu.2020.00697. eCollection 2020. Front Immunol. 2020. PMID: 32431696 Free PMC article. - Sex and sex steroids impact influenza pathogenesis across the life course.
Vom Steeg LG, Klein SL. Vom Steeg LG, et al. Semin Immunopathol. 2019 Mar;41(2):189-194. doi: 10.1007/s00281-018-0718-5. Epub 2018 Oct 8. Semin Immunopathol. 2019. PMID: 30298431 Free PMC article. Review. - Mechanisms of sex disparities in influenza pathogenesis.
Klein SL, Hodgson A, Robinson DP. Klein SL, et al. J Leukoc Biol. 2012 Jul;92(1):67-73. doi: 10.1189/jlb.0811427. Epub 2011 Nov 30. J Leukoc Biol. 2012. PMID: 22131346 Free PMC article. Review.
Cited by
- The Dual Faces of Oestrogen: The Impact of Exogenous Oestrogen on the Physiological and Pathophysiological Functions of Tissues and Organs.
Bartkowiak-Wieczorek J, Jaros A, Gajdzińska A, Wojtyła-Buciora P, Szymański I, Szymaniak J, Janusz W, Walczak I, Jonaszka G, Bienert A. Bartkowiak-Wieczorek J, et al. Int J Mol Sci. 2024 Jul 26;25(15):8167. doi: 10.3390/ijms25158167. Int J Mol Sci. 2024. PMID: 39125736 Free PMC article. Review. - Comparative study of the severity of Covid-19 infection between female and male patients.
Imzil A, Mansoury O, Oulahbib A, Adarmouch L, Serhane H. Imzil A, et al. Niger Med J. 2024 Apr 21;65(1):56-66. doi: 10.60787/nmj-v65i1-451. eCollection 2024 Jan-Feb. Niger Med J. 2024. PMID: 39006174 Free PMC article. - Mathematical Modeling Suggests That Monocyte Activity May Drive Sex Disparities during Influenza Infection.
Liparulo TS, Shoemaker JE. Liparulo TS, et al. Viruses. 2024 May 24;16(6):837. doi: 10.3390/v16060837. Viruses. 2024. PMID: 38932131 Free PMC article. - Sex Differences during Influenza A Virus Infection and Vaccination and Comparison of Cytokine and Antibody Responses between Plasma and Serum Samples.
Dhakal S, Wolfe BW, Pantha S, Vijayakumar S. Dhakal S, et al. Pathogens. 2024 Jun 1;13(6):468. doi: 10.3390/pathogens13060468. Pathogens. 2024. PMID: 38921766 Free PMC article. - Tissue-specific sex differences in pediatric and adult immune cell composition and function.
Mitul MT, Kastenschmidt JM, Sureshchandra S, Wagoner ZW, Sorn AM, Mcllwain DR, Hernandez-Davies JE, Jain A, de Assis R, Trask D, Davies DH, Wagar LE. Mitul MT, et al. Front Immunol. 2024 May 15;15:1373537. doi: 10.3389/fimmu.2024.1373537. eCollection 2024. Front Immunol. 2024. PMID: 38812520 Free PMC article.
References
- Klein SL, Huber S. Sex differences in susceptibility to viral infection. In: Klein SL, Roberts CW, editors. Sex hormones and immunity to infection. Berlin: Springer-Verlag; 2010. pp. 93–122.
- Marriott I, Huet-Hudson YM. Sexual dimorphism in innate immune responses to infectious organisms. Immunol Res. 2006;34:177–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 AI079342/AI/NIAID NIH HHS/United States
- AI089034/AI/NIAID NIH HHS/United States
- R21 AI090344/AI/NIAID NIH HHS/United States
- F31 AI089034/AI/NIAID NIH HHS/United States
- AI079342/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources