Probiotics modulate intestinal expression of nuclear receptor and provide counter-regulatory signals to inflammation-driven adipose tissue activation - PubMed (original) (raw)
Probiotics modulate intestinal expression of nuclear receptor and provide counter-regulatory signals to inflammation-driven adipose tissue activation
Andrea Mencarelli et al. PLoS One. 2011.
Abstract
Background: Adipocytes from mesenteric white adipose tissue amplify the inflammatory response and participate in inflammation-driven immune dysfunction in Crohn's disease by releasing proinflammatory mediators. Peroxisome proliferator-activated receptors (PPAR)-α and -γ, pregnane x receptor (PXR), farnesoid x receptor (FXR) and liver x-receptor (LXR) are ligand-activated nuclear receptor that provide counter-regulatory signals to dysregulated immunity and modulates adipose tissue.
Aims: To investigate the expression and function of nuclear receptors in intestinal and adipose tissues in a rodent model of colitis and mesenteric fat from Crohn's patients and to investigate their modulation by probiotics.
Methods: Colitis was induced by TNBS administration. Mice were administered vehicle or VSL#3, daily for 10 days. Abdominal fat explants obtained at surgery from five Crohn's disease patients and five patients with colon cancer were cultured with VSL#3 medium.
Results: Probiotic administration attenuated development of signs and symptoms of colitis, reduced colonic expression of TNFα, IL-6 and IFNγ and reserved colonic downregulation of PPARγ, PXR and FXR caused by TNBS. Mesenteric fat depots isolated from TNBS-treated animals had increased expression of inflammatory mediators along with PPARγ, FXR, leptin and adiponectin. These changes were prevented by VSL#3. Creeping fat and mesenteric adipose tissue from Crohn's patients showed a differential expression of PPARγ and FXR with both tissue expressing high levels of leptin. Exposure of these tissues to VSL#3 medium abrogates leptin release.
Conclusions: Mesenteric adipose tissue from rodent colitis and Crohn's disease is metabolically active and shows inflammation-driven regulation of PPARγ, FXR and leptin. Probiotics correct the inflammation-driven metabolic dysfunction.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. Anti-inflammatory activity of VSL#3 in TNBS colitis.
Preteatment with VSL#3 (50×109 colony-forming units (cfu)/kg/day) protects against the development of TNBS-induced colitis in mice. Colitis was induced by intrarectal instillation of 1.5 mg of TNBS per mouse. Mice were sacrificed 5 days after TNBS administration. (A and B) The severity of TNBS-induced inflammation (weight loss and fecal score) is reduced by VSL#3 administration. Data represent the mean ± SE of 8–10 mice per group. (#p<0.05 versus naïve; *p<0.05 versus TNBS). (C and D) VSL#3 reduces local signs of inflammation and inhibits the increase of macroscopic-score and neutrophil infiltration (MPO activity) induced by TNBS. Data represent the mean ± SE of 8–10 mice per group. (#p<0.05 versus naïve; *p<0.05 versus TNBS).
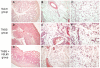
Figure 2. Histological analysis of colon and mesenteric adipose tissue of mice treated with TNBS alone or in combination with VSL#3.
(A, D and G) Histopathology analysis of colon samples, original magnification 10×; H&E staining. (A) naïve mice; (D) TNBS administration causes colon wall thickening and massive inflammatory infiltration in the lamina propria and mucosal erosions_;_ (G) VSL#3 attenuates colon thickening and inflammatory infiltration of the mucosa and submucosa. (B,C,E,F,H and I) Histologic analysis of mesenteric adipose tissue, H&E staining. (B and C) Naïve mice, original magnification 10× and 20×; (E and F) TNBS group mice, original magnification 10× and 20×; (H and I) VSL#3 treated mice, original magnification 10× and 20x.
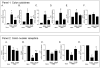
Figure 3. VSL#3 attenuates inflammatory changes in the colon and restores nuclear receptors expression in mice administered TNBS.
(Panel 1. A–F) RT-PCR analysis of the expression of inflammatory cytokines (IL-6, TNFα, IL-1β, and INFγ) and anti-inflammatory cytokines (IL-10 and TGF-β) in colons obtained 5 days after TNBS. Data represent the mean ± SE of 5 mice per group. (#p<0.05 versus naïve; *p<0.05 versus TNBS). (Panel 2 A–E) RT-PCR analysis of the expression of PPARα, PPARγ, FXR, LXR, PXR and CAR in colons removed 5 days after administration of TNBS alone or in combination with VSL#3. Data represent the mean ± SE of 5 mice per group. (#p<0.05 versus naïve; *p<0.05 versus TNBS).
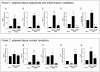
Figure 4. VSL#3 attenuates inflammation-driven metabolic dysfunction in the mesenteric adipose tissue.
(Panel 1 A-E) RT-PCR analysis of expression of inflammatory cytokines (TNFα, IL-6, and MCP1) and adipokines (leptin and adiponectin) in mesenteric adipose tissues. Data represent the mean ± SE of 5 mice per group. (#p<0.05 versus naïve; *p<0.05 versus TNBS). (Panel 2 A-E) RT-PCR analysis of the expression of PPARα, PPARγ, LXR, PXR and FXR in mesenteric adipose tissues obtained 5 days after administration of TNBS alone or in combination with VSL#3. Data represent the mean ± SE of 5 mice per group. (#p<0.05 versus naïve; *p<0.05 versus TNBS).
Figure 5. Distinctive histologic features the creeping fat and mesenteric adipose tissue in Crohn's disease patients.
Rappresentative haematoxylin-eosin (H&E) staining of mesenteric adipose tissue of Crohn's patients. Creeping fat original magnification 10× and 20×, respectively (A) adipose tissue distal to intestinal mucosa of Crohn's disease patients, original magnification 20× (Bars: 100 µm) and 40×(Bars: 50 µm), respectively (B). Paired samples from three patients are shown.
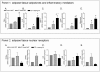
Figure 6. Expression of inflammatory mediators, adipokines and selected nuclear receptors in mesenteric adipose tissues from Crohn's patients.
RT-PC analysis of expression of inflammatory TNFα, IL-6, MCP1, leptin and adiponectin (Panel 1 A-E) and nuclear receptors (PPARα, PPARγ, LXR, PXR and FXR) (Panel 2 A-E) in mesenteric adipose tissue (MAT) obtained from control subjects (N = 5) (colon carcinoma) and in Crohn's patients (creeping fat and distal MAT) (N = 5). Data represent the mean ± SE of 5 different subjects per group. (#p<0.05 versus MAT of control subjects ; *p<0.05 versus distal MAT obtained from Crohn's disease patients).
Figure 7. VSL#3 CM modulates the production of mesenteric adipose tissue factors.
Release of adiponectin, leptin, IL-6 and TNFα by adipose tissue explants. Release by proximal (creeping fat) and distal MAT explants from 5 Crhon's patients is shown. Creeping fat and MAT explants were cultured alone or in combination with different concentrations of VSL#3 CM for 48 h. (#p<0.05 basal production (Ctrl) creeping versus MAT; n = 25; * p<0.05 verus control group, n = 25).
Similar articles
- Infliximab modifies mesenteric adipose tissue alterations and intestinal inflammation in rats with TNBS-induced colitis.
Clemente TR, Dos Santos AN, Sturaro JN, Gotardo EM, de Oliveira CC, Acedo SC, Caria CR, Pedrazzoli J Jr, Ribeiro ML, Gambero A. Clemente TR, et al. Scand J Gastroenterol. 2012 Sep;47(8-9):943-50. doi: 10.3109/00365521.2012.688213. Epub 2012 May 28. Scand J Gastroenterol. 2012. PMID: 22630819 - Nuclear receptors and nonalcoholic fatty liver disease.
Cave MC, Clair HB, Hardesty JE, Falkner KC, Feng W, Clark BJ, Sidey J, Shi H, Aqel BA, McClain CJ, Prough RA. Cave MC, et al. Biochim Biophys Acta. 2016 Sep;1859(9):1083-1099. doi: 10.1016/j.bbagrm.2016.03.002. Epub 2016 Mar 4. Biochim Biophys Acta. 2016. PMID: 26962021 Free PMC article. Review. - Gene expression profiling identifies mechanisms of protection to recurrent trinitrobenzene sulfonic acid colitis mediated by probiotics.
Mariman R, Kremer B, van Erk M, Lagerweij T, Koning F, Nagelkerken L. Mariman R, et al. Inflamm Bowel Dis. 2012 Aug;18(8):1424-33. doi: 10.1002/ibd.22849. Epub 2011 Dec 11. Inflamm Bowel Dis. 2012. PMID: 22162025 - Effects of obesity on severity of colitis and cytokine expression in mouse mesenteric fat. Potential role of adiponectin receptor 1.
Sideri A, Stavrakis D, Bowe C, Shih DQ, Fleshner P, Arsenescu V, Arsenescu R, Turner JR, Pothoulakis C, Karagiannides I. Sideri A, et al. Am J Physiol Gastrointest Liver Physiol. 2015 Apr 1;308(7):G591-604. doi: 10.1152/ajpgi.00269.2014. Epub 2015 Jan 15. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25591865 Free PMC article.
Cited by
- Discovery of a Novel Multi-Strains Probiotic Formulation with Improved Efficacy toward Intestinal Inflammation.
Biagioli M, Carino A, Di Giorgio C, Marchianò S, Bordoni M, Roselli R, Distrutti E, Fiorucci S. Biagioli M, et al. Nutrients. 2020 Jun 30;12(7):1945. doi: 10.3390/nu12071945. Nutrients. 2020. PMID: 32629887 Free PMC article. - THE THERAPEUTIC IMPACT OF PROBIOTICS ON NONALCOHOLIC FATTY LIVER DISEASE IN PEDIATRICS: A SYSTEMATIC REVIEW.
Chaves FGB, Oliveira GF, Ribeiro JP, Serafim JV, Cordeiro LFM, Alvares MA, Cecchi MT, Vasquez MC, Souza TBD, Rullo VEV. Chaves FGB, et al. Rev Paul Pediatr. 2021;39:e2019226. doi: 10.1590/1984-0462/2021/39/2019226. Epub 2020 Aug 26. Rev Paul Pediatr. 2021. PMID: 32876312 Free PMC article. - Urinary (1)H-NMR-based metabolic profiling of children with NAFLD undergoing VSL#3 treatment.
Miccheli A, Capuani G, Marini F, Tomassini A, Praticò G, Ceccarelli S, Gnani D, Baviera G, Alisi A, Putignani L, Nobili V. Miccheli A, et al. Int J Obes (Lond). 2015 Jul;39(7):1118-25. doi: 10.1038/ijo.2015.40. Epub 2015 Mar 26. Int J Obes (Lond). 2015. PMID: 25809828 Clinical Trial. - A review on the potential of underutilized Blackjack (Biden Pilosa) naturally occurring in sub-Saharan Africa.
Mtenga DV, Ripanda AS. Mtenga DV, et al. Heliyon. 2022 May 29;8(6):e09586. doi: 10.1016/j.heliyon.2022.e09586. eCollection 2022 Jun. Heliyon. 2022. PMID: 35669545 Free PMC article. Review. - The bile acid sensor FXR is required for immune-regulatory activities of TLR-9 in intestinal inflammation.
Renga B, Mencarelli A, Cipriani S, D'Amore C, Carino A, Bruno A, Francisci D, Zampella A, Distrutti E, Fiorucci S. Renga B, et al. PLoS One. 2013;8(1):e54472. doi: 10.1371/journal.pone.0054472. Epub 2013 Jan 25. PLoS One. 2013. PMID: 23372731 Free PMC article.
References
- Sheehan AL, Warren BF, Gear MW, Shepherd NA. Fat-wrapping in Crohn's disease: pathological basis and relevance to surgical practice. Br J Surg. 1992;79:955–958. - PubMed
- Smedh K, Olaison G, Nyström PO, Sjödahl R. Intraoperative enteroscopy in Crohn's disease. Br J Surg. 1993;80:897–900. - PubMed
- Borley NR, Mortensen NJ, Jewell DP, Warren BF. The relationship between inflammatory and serosal connective tissue changes in ileal Crohn's disease: evidence for a possible causative link. J Pathol. 2000;190:196–202. - PubMed
- Fazio VW, Jones IT. Standard surgical treatment of Crohn's disease of the small intestine and ileocolitis. In: Lee ECG, Nolan DJ, eds. Surgery of inflammatory bowel disorders. Edinburgh: Churchill Livingstone. 1987;147–56
- Paul G, Schäffler A, Neumeier M, Fürst A, Bataillle F, et al. Profiling adipocytokine secretion from creeping fat in Crohn's disease. Inflamm Bowel Dis. 2006;12:471–477. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources