Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? - PubMed (original) (raw)
doi: 10.1186/1742-2094-8-94.
Martin Walter, Tomasz Gos, Gilles J Guillemin, Hans-Gert Bernstein, Zoltán Sarnyai, Christian Mawrin, Ralf Brisch, Hendrik Bielau, Louise Meyer zu Schwabedissen, Bernhard Bogerts, Aye-Mu Myint
Affiliations
- PMID: 21831269
- PMCID: PMC3177898
- DOI: 10.1186/1742-2094-8-94
Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission?
Johann Steiner et al. J Neuroinflammation. 2011.
Erratum in
- J Neuroinflammation. 2013;10:34
Abstract
Background: Immune dysfunction, including monocytosis and increased blood levels of interleukin-1, interleukin-6 and tumour necrosis factor α has been observed during acute episodes of major depression. These peripheral immune processes may be accompanied by microglial activation in subregions of the anterior cingulate cortex where depression-associated alterations of glutamatergic neurotransmission have been described.
Methods: Microglial immunoreactivity of the N-methyl-D-aspartate (NMDA) glutamate receptor agonist quinolinic acid (QUIN) in the subgenual anterior cingulate cortex (sACC), anterior midcingulate cortex (aMCC) and pregenual anterior cingulate cortex (pACC) of 12 acutely depressed suicidal patients (major depressive disorder/MDD, n = 7; bipolar disorder/BD, n = 5) was analyzed using immunohistochemistry and compared with its expression in 10 healthy control subjects.
Results: Depressed patients had a significantly increased density of QUIN-positive cells in the sACC (P = 0.003) and the aMCC (P = 0.015) compared to controls. In contrast, counts of QUIN-positive cells in the pACC did not differ between the groups (P = 0.558). Post-hoc tests showed that significant findings were attributed to MDD and were absent in BD.
Conclusions: These results add a novel link to the immune hypothesis of depression by providing evidence for an upregulation of microglial QUIN in brain regions known to be responsive to infusion of NMDA antagonists such as ketamine. Further work in this area could lead to a greater understanding of the pathophysiology of depressive disorders and pave the way for novel NMDA receptor therapies or immune-modulating strategies.
Figures
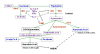
Figure 1
modified from [13]: Tryptophan is an essential amino acid and a precursor for the synthesis of serotonin. Alternatively, tryptophan can be metabolized in glial cells via the kynurenine pathway to create kynurenic acid (synthesized by kynurenine aminotransferase, KAT) or quinolinic acid (QUIN). These substances are endogenous modulators of NMDA glutamate receptors. A key enzyme of the kynurenine pathway, indoleamine 2,3-dioxygenase (IDO), and the enzyme that catalyses the production of 3-OH-kynurenine, kynurenine monoxygenase (KMO), are activated by proinflammatory cytokines, including interleukin-1 and -6 (IL-1, IL-6), tumor necrosis factor a (TNFa), or interferon g (IFNg). These enzymes are inhibited by anti-inflammatory cytokines, including IL-4. Serotonin is normally broken down into 5-hydroxyindoleacetic acid (5-HIAA), but the indole ring of serotonin can also be cleaved by IDO to form formyl-5-hydroxykynurenamine (f-5-KYM). Annotation: grey arrows: activation; dotted grey lines with bar at the end: inhibition; black font: potentially neurotoxic; purple font: neutral or not known; bright blue: potentially neuroprotective.
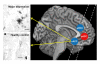
Figure 2
Illustrations of QUIN-immunoreactive cells from the left sACC of a depressed suicidal patient and a control case and the locations of the analyzed regions of interest (sACC, aMCC and pACC). Depressed patients showed microglial formations with numerous granular structure processes. Annotation: Scale bars represent 20 μm.
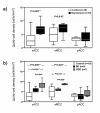
Figure 3
Illustration of QUIN-immunopositive cell densities. a) Depressed patients had increased QUIN-immunopositive cell densities in the sACC and the aMCC but not in the pACC. b) MDD patients showed the highest QUIN-immunoreactive cell counts in the sACC and the aMCC compared to BD and control cases. No diagnostic subgroup-dependent differences were observed in the pACC. Annotation: The box plots show the median, interquartile range, sample minimum and sample maximum, * P < 0.05, ** P < 0.01.
Similar articles
- Decreased quinolinic acid in the hippocampus of depressive patients: evidence for local anti-inflammatory and neuroprotective responses?
Busse M, Busse S, Myint AM, Gos T, Dobrowolny H, Müller UJ, Bogerts B, Bernstein HG, Steiner J. Busse M, et al. Eur Arch Psychiatry Clin Neurosci. 2015 Jun;265(4):321-9. doi: 10.1007/s00406-014-0562-0. Epub 2014 Nov 20. Eur Arch Psychiatry Clin Neurosci. 2015. PMID: 25409655 - Regional metabolic heterogeneity in anterior cingulate cortex in major depressive disorder: A multi-voxel 1H magnetic resonance spectroscopy study.
He J, Wang D, Ban M, Kong L, Xiao Q, Yuan F, Zhu X. He J, et al. J Affect Disord. 2022 Dec 1;318:263-271. doi: 10.1016/j.jad.2022.09.001. Epub 2022 Sep 7. J Affect Disord. 2022. PMID: 36087788 - Reduced anterior cingulate glutamatergic concentrations in childhood OCD and major depression versus healthy controls.
Rosenberg DR, Mirza Y, Russell A, Tang J, Smith JM, Banerjee SP, Bhandari R, Rose M, Ivey J, Boyd C, Moore GJ. Rosenberg DR, et al. J Am Acad Child Adolesc Psychiatry. 2004 Sep;43(9):1146-53. doi: 10.1097/01.chi.0000132812.44664.2d. J Am Acad Child Adolesc Psychiatry. 2004. PMID: 15322418 - The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression.
Müller N, Schwarz MJ. Müller N, et al. Mol Psychiatry. 2007 Nov;12(11):988-1000. doi: 10.1038/sj.mp.4002006. Epub 2007 Apr 24. Mol Psychiatry. 2007. PMID: 17457312 Review. - Cingulate role in Tourette syndrome.
O'Neill J, Piacentini JC, Peterson BS. O'Neill J, et al. Handb Clin Neurol. 2019;166:165-221. doi: 10.1016/B978-0-444-64196-0.00011-X. Handb Clin Neurol. 2019. PMID: 31731911 Review.
Cited by
- Mechanism of N6-Methyladenosine Modification in the Pathogenesis of Depression.
Xian Z, Tian L, Yao Z, Cao L, Jia Z, Li G. Xian Z, et al. Mol Neurobiol. 2024 Nov 18. doi: 10.1007/s12035-024-04614-6. Online ahead of print. Mol Neurobiol. 2024. PMID: 39551913 Review. - Tryptophan Metabolism Disorder-Triggered Diseases, Mechanisms, and Therapeutic Strategies: A Scientometric Review.
Chen X, Xu D, Yu J, Song XJ, Li X, Cui YL. Chen X, et al. Nutrients. 2024 Oct 4;16(19):3380. doi: 10.3390/nu16193380. Nutrients. 2024. PMID: 39408347 Free PMC article. Review. - The major biogenic amine metabolites in mood disorders.
Yang J, Yuan M, Zhang W. Yang J, et al. Front Psychiatry. 2024 Sep 24;15:1460631. doi: 10.3389/fpsyt.2024.1460631. eCollection 2024. Front Psychiatry. 2024. PMID: 39381610 Free PMC article. Review. - Tofacitinib prevents depressive-like behaviors through decreased hippocampal microgliosis and increased BDNF levels in both LPS-induced and CSDS-induced mice.
Gao YN, Pan KJ, Zhang YM, Qi YB, Chen WG, Zhou T, Zong HC, Guo HR, Zhao JW, Liu XC, Cao ZT, Chen Z, Yin T, Zang Y, Li J. Gao YN, et al. Acta Pharmacol Sin. 2024 Sep 30. doi: 10.1038/s41401-024-01384-8. Online ahead of print. Acta Pharmacol Sin. 2024. PMID: 39349767 - Neurodevelopmental and Neuropsychiatric Disorders.
Traetta ME, Chaves Filho AM, Akinluyi ET, Tremblay MÈ. Traetta ME, et al. Adv Neurobiol. 2024;37:457-495. doi: 10.1007/978-3-031-55529-9_26. Adv Neurobiol. 2024. PMID: 39207708 Review.
References
- Kaestner F, Hettich M, Peters M, Sibrowski W, Hetzel G, Ponath G, Arolt V, Cassens U, Rothermundt M. Different activation patterns of proinflammatory cytokines in melancholic and non-melancholic major depression are associated with HPA axis activity. J Affect Disord. 2005;87:305–311. doi: 10.1016/j.jad.2005.03.012. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources