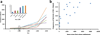A role for systems epidemiology in tuberculosis research - PubMed (original) (raw)
Review
A role for systems epidemiology in tuberculosis research
Iñaki Comas et al. Trends Microbiol. 2011 Oct.
Abstract
Despite being a curable disease, tuberculosis (TB) killed more people in 2009 than during any previous year in history. Progress in TB research has been slow, and remains burdened by important gaps in our knowledge of the basic biology of Mycobacterium tuberculosis, the causative agent of TB, and its interaction with the human host. Fortunately, major systems biology initiatives have recently been launched that will help fill some of these gaps. However, to fully comprehend TB and control this disease globally, current systems biological approaches will not suffice. The influence of host and pathogen diversity, changes in human demography, and socioeconomic and environmental factors will also need to be considered. Such a multidisciplinary approach might be best described as 'systems epidemiology' in an effort to overcome the traditional boundaries between basic biology and classical epidemiology.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
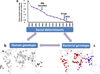
Figure 1. MTBC strain variation and the spectrum of responses to TB
Instead of the traditional binary division into active and latent TB, a spectrum of patient responses to infection has been proposed to better describe the observed heterogeneity in TB patients and latently infected individuals [7]. HIV-coinfection and increased bacterial burden drive latency towards active disease (adapted from [7]). Likewise, pathogen genotype could also play a role in shaping the outcome of TB within this spectrum. For example, based on recent data, ‘modern’ MTBC lineages elicit a delayed innate immune response [59], and progress faster to active disease [62] compared to ‘ancient’ strains.
Figure 2. TB driven by biology and sociology
Many environmental, socioeconomic and evolutionary factors are not generally considered when applying current systems biological approaches to infectious disease research. (a) Improved living conditions lead to a decline in TB deaths. In England and Wales TB mortality started to decrease long before the causative agent of TB (Mtb) was identified by Robert Koch and before BCG vaccination and chemotherapy became available (adapted from [27]). (b) Human genetic diversity impacts susceptibility to TB. The HLA II allele DQB1*0503 was the first HLA associated to increase TB risk [80] and along with other DQB1 alleles has been associated with increase susceptibility to TB in different places in Asia [31]. The DQB1*0503 allele is more common in Asian countries and pacific islands (dark grey and black dots) than other parts of the world (light grey) (data from
http://www.allelefrequencies.net
, Box 2). (c) Geographical distribution of the six human MTBC lineages. Each dot represents a country and its color indicates the most frequent lineage(s) within this country (adapted from [42]). The three ‘modern’ lineages (Lineage 4, red; Lineage 2, blue; Lineage 3, purple) are more globally widespread and hence more successful compared to the three ‘ancient’ lineages (M. africanum Lineage 5, green; M. africanum Lineage 6, brown; Lineage 1, pink), which are more geographically restricted. There is increasing evidence for a role of bacterial genotype in TB infection and disease [32].
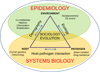
Figure 3. Systems epidemiology of TB
Systems biology will be key to elucidate the biology of TB. In addition, complementary efforts need to be directed towards understanding the environmental, sociological and evolutionary factors driving global TB epidemics (adapted from [26]).
Figure 4. Experimental and epidemiological evidence for the impact of bacterial genotype on TB disease
(a) ‘Modern’ lineages elicit lower pro-inflammatory cytokines (e.g. IL-6) compared to ‘ancient’ lineages in human monocyte-derived macrophages [59]. The inhibition of innate immunity could allow ‘modern’ strains to replicate and establish an infection more efficiently before more efficient immune responses quick in. Inhibition of innate immunity has been associated with increased MTBC virulence in animal models of infection [60, 61]. (b) Strains of the ‘ancient’ Lineage 6, also known as M. africanum, were three times less likely to progress to active TB compared to other MTBC lineages [62].
Figure I. Human demographic changes and the evolution of TB
(a) For most of human history, the population of the world was less than one billion people. By the end of the 20th century, the population was six billion, and it is estimated that by 2050 it will reach 10 billion. This dramatic increase in human population occurred unevenly across different geographic regions, which could have influenced the evolution of the different MTBC lineages. ‘Modern’ lineages evolved in high populated areas whereas ‘ancient’ lineages have been evolving in regions where human densities remained low until very recently. However, these regions are catching up and expected to experience the strongest population growth in the future (data from
http://www.sasi.group.shef.ac.uk/worldmapper
, Box 2). (b) In addition to these historical and projected trends of population growth, changes in human behaviours that lead to higher population densities could also affect the evolution of the pathogen and the host. Humans with a deletion in the gene SLC11A1 are more resistant to intracellular infections. The frequency of this gene variant is positively correlated with regions of early urbanization [78]. Urbanization leads to an increase in human densities, which could favour the evolution of more virulent strains [79].
Similar articles
- Host-pathogen coevolution in human tuberculosis.
Gagneux S. Gagneux S. Philos Trans R Soc Lond B Biol Sci. 2012 Mar 19;367(1590):850-9. doi: 10.1098/rstb.2011.0316. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 22312052 Free PMC article. Review. - The arms race between man and Mycobacterium tuberculosis: Time to regroup.
Hoal EG, Dippenaar A, Kinnear C, van Helden PD, Möller M. Hoal EG, et al. Infect Genet Evol. 2018 Dec;66:361-375. doi: 10.1016/j.meegid.2017.08.021. Epub 2017 Aug 23. Infect Genet Evol. 2018. PMID: 28843547 Review. - Dissecting Host-Pathogen Interactions in TB Using Systems-Based Omic Approaches.
Borah K, Xu Y, McFadden J. Borah K, et al. Front Immunol. 2021 Nov 2;12:762315. doi: 10.3389/fimmu.2021.762315. eCollection 2021. Front Immunol. 2021. PMID: 34795672 Free PMC article. Review. - Mathematical modelling of the epidemiology of tuberculosis.
White PJ, Garnett GP. White PJ, et al. Adv Exp Med Biol. 2010;673:127-40. doi: 10.1007/978-1-4419-6064-1_9. Adv Exp Med Biol. 2010. PMID: 20632534 Review. - Evolution of virulence in the Mycobacterium tuberculosis complex.
Orgeur M, Brosch R. Orgeur M, et al. Curr Opin Microbiol. 2018 Feb;41:68-75. doi: 10.1016/j.mib.2017.11.021. Epub 2017 Dec 5. Curr Opin Microbiol. 2018. PMID: 29216510 Review.
Cited by
- Investigation of miRNA and cytokine expressions in latent tuberculosis infection and active tuberculosis.
Çavuşoğlu C, Çoğulu Ö, Durmaz A, Cengisiz Z, Yılmaz FF, Taşbakan MS, Taşbakan M, Gündüz C, Biçmen C, Karaman O, Taşlıdere H, Akın H, Akarca T, Dereli Ş. Çavuşoğlu C, et al. Turk J Med Sci. 2022 Jun;52(3):649-657. doi: 10.55730/1300-0144.5357. Epub 2022 Jun 16. Turk J Med Sci. 2022. PMID: 36326316 Free PMC article. - Translating basic science insight into public health action for multidrug- and extensively drug-resistant tuberculosis.
Walter ND, Strong M, Belknap R, Ordway DJ, Daley CL, Chan ED. Walter ND, et al. Respirology. 2012 Jul;17(5):772-91. doi: 10.1111/j.1440-1843.2012.02176.x. Respirology. 2012. PMID: 22458269 Free PMC article. Review. - Tackling Drug-Resistant Tuberculosis: New Challenges from the Old Pathogen Mycobacterium tuberculosis.
Mancuso G, Midiri A, De Gaetano S, Ponzo E, Biondo C. Mancuso G, et al. Microorganisms. 2023 Sep 10;11(9):2277. doi: 10.3390/microorganisms11092277. Microorganisms. 2023. PMID: 37764122 Free PMC article. Review. - Impact of pe_pgrs33 Gene Polymorphisms on Mycobacterium tuberculosis Infection and Pathogenesis.
Camassa S, Palucci I, Iantomasi R, Cubeddu T, Minerva M, De Maio F, Jouny S, Petruccioli E, Goletti D, Ria F, Sali M, Sanguinetti M, Manganelli R, Rocca S, Brodin P, Delogu G. Camassa S, et al. Front Cell Infect Microbiol. 2017 Apr 21;7:137. doi: 10.3389/fcimb.2017.00137. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28484686 Free PMC article. - Spoligotyping and whole-genome sequencing analysis of lineage 1 strains of Mycobacterium tuberculosis in Da Nang, Vietnam.
Hijikata M, Keicho N, Duc LV, Maeda S, Hang NTL, Matsushita I, Kato S. Hijikata M, et al. PLoS One. 2017 Oct 19;12(10):e0186800. doi: 10.1371/journal.pone.0186800. eCollection 2017. PLoS One. 2017. PMID: 29049400 Free PMC article.
References
- Smith NH, et al. Ecotypes of the Mycobacterium tuberculosis complex. J Theor Biol. 2006;239:220–225. - PubMed
- World Health Organization. Geneva: WHO; 2010. Global tuberculosis control - surveillance, planning, financing.
- Dye C, Williams BG. The population dynamics and control of tuberculosis. Science. 2010;328:856–861. - PubMed
- Young D, et al. Systems biology of persistent infection: tuberculosis as a case study. Nat Rev Microbiol. 2008;6:520–528. - PubMed
Publication types
MeSH terms
Grants and funding
- MRC_U117588500/MRC_/Medical Research Council/United Kingdom
- R01 AI090928-01/AI/NIAID NIH HHS/United States
- AI090928/AI/NIAID NIH HHS/United States
- MC_U117588500/MRC_/Medical Research Council/United Kingdom
- N01 AI070022/AI/NIAID NIH HHS/United States
- HHSN266200700022C/PHS HHS/United States
- AA080019/AA/NIAAA NIH HHS/United States
- R01 AI090928/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical