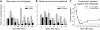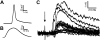Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. I. Loss of VGLUT1/IA synapses on motoneurons - PubMed (original) (raw)
Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. I. Loss of VGLUT1/IA synapses on motoneurons
Francisco J Alvarez et al. J Neurophysiol. 2011 Nov.
Abstract
Motor and sensory proprioceptive axons reinnervate muscles after peripheral nerve transections followed by microsurgical reattachment; nevertheless, motor coordination remains abnormal and stretch reflexes absent. We analyzed the possibility that permanent losses of central IA afferent synapses, as a consequence of peripheral nerve injury, are responsible for this deficit. VGLUT1 was used as a marker of proprioceptive synapses on rat motoneurons. After nerve injuries synapses are stripped from motoneurons, but while other excitatory and inhibitory inputs eventually recover, VGLUT1 synapses are permanently lost on the cell body (75-95% synaptic losses) and on the proximal 100 μm of dendrite (50% loss). Lost VGLUT1 synapses did not recover, even many months after muscle reinnervation. Interestingly, VGLUT1 density in more distal dendrites did not change. To investigate whether losses are due to VGLUT1 downregulation in injured IA afferents or to complete synaptic disassembly and regression of IA ventral projections, we studied the central trajectories and synaptic varicosities of axon collaterals from control and regenerated afferents with IA-like responses to stretch that were intracellularly filled with neurobiotin. VGLUT1 was present in all synaptic varicosities, identified with the synaptic marker SV2, of control and regenerated afferents. However, regenerated afferents lacked axon collaterals and synapses in lamina IX. In conjunction with the companion electrophysiological study [Bullinger KL, Nardelli P, Pinter MJ, Alvarez FJ, Cope TC. J Neurophysiol (August 10, 2011). doi:10.1152/jn.01097.2010], we conclude that peripheral nerve injuries cause a permanent retraction of IA afferent synaptic varicosities from lamina IX and disconnection with motoneurons that is not recovered after peripheral regeneration and reinnervation of muscle by sensory and motor axons.
Figures
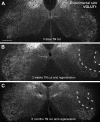
Fig. 1.
VGLUT1 immunoreactivity is permanently depleted in lamina IX regions containing motoneurons and central projections of IA afferents injured peripherally. A: low-magnification confocal image of VGLUT1 immunoreactivity in the ventral horns of the spinal cord in a rat with a unilateral tibial nerve (TN) transection performed 3 days earlier. VGLUT1-immunoreactive (IR) puncta are very dense in the lateral regions of lamina IX. VGLUT1-IR puncta at this location preferentially label the synaptic varicosities of IA afferents. No differences can be appreciated between the sides at this short postinjury time. B: image similar to A but from a rat whose TN was transected 2 wk earlier and allowed to regenerate and reinnervate the muscles. A large depletion is observed in the region corresponding to motoneuron pools sending axons through the TN (region marked between dashed lines). C: same as in B, but in an animal that fully regenerated its peripheral muscle 6 mo after the injury. VGLUT1-IR puncta remained depleted around TN motor pools in lamina IX (region between dashed lines). Scale bar in A is 250 μm. All images are at the same magnification.
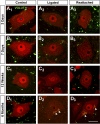
Fig. 2.
VGLUT1-IR contacts on neuronal nuclear protein (NeuN)-IR motoneuron cell bodies are permanently depleted after nerve injury and do not recover after muscle reinnervation. A–D: high-magnification, single plane, confocal images of VGLUT1-IR varicosities (FITC, green) and NeuN-IR cell bodies (Cy3, red) sampled from lamina IX regions containing motoneurons with axons injured in the TN at various times after injury: 3 days (A), 7 days (B), 12 wk (C), and 6 mo (D). Control motoneurons (A1, B1, C1, D1) show strong NeuN immunoreactivity in the cell nucleus and are surrounded by VGLUT1 varicosities in the neuropil, some of which are in contact with the cell body. Injured motoneurons whose axons are not allowed to regenerate (ligated; A2, B2, C2, D2) show progressive downregulation of NeuN, especially in the cell nucleus. VGLUT1 varicosities are reduced in density in the adjacent neuropil, and they make fewer contacts with motoneuron cell bodies. VGLUT1-IR varicosities also appear smaller. Injured motoneurons whose axons have been rejoined in the periphery and are undergoing regeneration (A3, B3) also lose NeuN immunoreactivity initially, but this is recovered after reconnection with muscle (C3, D3). VGLUT1-IR varicosities are depleted and reduced in size on regenerating (A3, B3) or reconnected (C3, D3) motoneurons to a degree similar to that observed on motoneurons that do not regenerate. Motoneurons in older animals (usually at longer survival times) display autofluorescent lipofucsin granules in their somata (arrowheads in D series). Scale bar in D3 is 25 μm. All other panels are at the same magnification.
Fig. 3.
Reductions on VGLUT1 contact density on the cell bodies of NeuN-IR motoneurons axotomized in the periphery are quantitatively similar when axon regeneration is prevented or allowed. A: VGLUT1-IR contact densities around NeuN-IR motoneuron cell bodies after nerve cut and ligation to prevent regeneration at different postinjury times. Pairs of gray (control side) and black (experimental side) bars are shown for each animal at each survival day. Progressive depletions occur in the experimental side compared with the control side 1 and 2 wk after injury (7 and 14 days, respectively). This depletion becomes very large from 4 wk (28 days) to 6 mo (168 days) after injury [2 animals were analyzed 12 wk (84 days) after injury]. Asterisks denote significant differences when comparing control and experimental sides by t_-tests (*P < 0.05, **P < 0.01, ***P < 0.001). B: similar to A, but from animals in which the TN was rejoined and allowed to regenerate. As before, VGLUT1 depletions in the experimental side are progressive in the first 2 wk and then very profound from 4 wk to 6 mo, with no evidence of recovery. Significant variability was observed in the control sides of the different animals analyzed in A and B. This might represent normal intrinsic variability in the number of contacts reaching the cell body in different animals, a possible sampling bias in immunoreactions necessarily carried out at different times in different animals, or an adaptation after injury in the control side. However, on average the control side of all animals in the nerve ligation experiments was not significantly different from control animals undergoing regeneration or from the estimated average densities in 4 animals with no nerve injuries (see Fig. 5_A). C: % depletions within each animal comparing average VGLUT1 densities in control vs. experimental sides (the point at 84 days for cut and ligation contains average depletions calculated in 2 animals; for all other points, each point represents 1 animal). A similar sharp decline in VGLUT1 contacts on the cell soma of axotomized motoneurons, either regenerating (grey dashed line, open circles) or not (black line and filled circles), occurs during the first 2 wk. The % depletion plateaus at 4 wk, and there is no significant recovery in animals that regenerate and reconnect with muscle or in those prevented from regeneration.
Fig. 4.
Dual retrograde labeling for identification of medial gastrocnemius (MG) motoneurons that reinnervate the MG muscle. A: experimental design. The left MG muscle was injected with Fast Blue (1) prior to transection and reattachment of the TN (2). Then 1 wk before the end of the survival period (6 wk or 6 mo) the MG muscle was injected again (3) with cholera toxin b coupled to Alexa 555 (CTb-555). B and C: low-magnification confocal images (all optical planes through a 50-μm-thick section were superimposed) of Fast Blue (FB) and CTb-555 retrograde labeling from the MG, respectively, before and after the nerve lesion (from a 6 wk survival animal). B shows the location of labeled motoneurons within lamina (L)IX in a lumbar 5 segment section counterstained with fluorescent Nissl (gray, 640-Neurotrace) to delineate lamination (dashed blue lines). The yellow line indicates the boundary between the gray and white matter [central canal (CC)]. Motoneurons were labeled either for Fast Blue or CTb-555 only or dual labeled. Dual-labeled motoneurons (pink, asterisk) represent MG motoneurons confirmed as reinnervating the MG muscle. Neurons labeled only with Fast Blue or CTb-555 might be MG motoneurons that failed to uptake the tracer in one of the two injections or, alternatively, represent MG motoneurons reinnervating a different muscle after regeneration (Fast Blue) or non-MG motoneurons that after regeneration reinnervate the MG muscle (CTb-555). The analysis compared Fast Blue only vs. dual-labeled motoneurons since there were relatively few motoneurons labeled only with CTb in most experiments. C shows the same section but with VGLUT1 immunoreactivity superimposed (FITC, green). As before, a large depletion in VGLUT1 varicosities is found 6 wk after injury in the lamina IX region (dotted yellow outline) occupied by motoneurons and the central arborizations of IA afferents with peripheral axons in the injured TN. MG motoneurons are located at relatively mid-dorsoventral positions within this region (blue dashed line indicates the lamina IX boundary from B). D: high-magnification image of retrogradely labeled motoneurons showing VGLUT1 contacts on dendrites and cell bodies. Scale bars: 250 μm in C (B is at the same magnification), 50 μm in D.
Fig. 5.
Quantitative analysis of changes in VGLUT1-IR contacts on the cell bodies of MG motoneurons at different postinjury times after tibial or MG nerve transections and reunion. A: average VGLUT1-IR contact density on CTb-labeled (control and 1 wk) or Fast Blue and CTb dual-labeled MG motoneurons (6 wk and 6 mo) in individual animals (6–14 motoneurons analyzed per animal) from the control (black bars), 1 wk survival (medium gray bars), 6 wk (dark gray bars), and 6 mo (light gray bars) groups. Despite some interanimal variability (depicted as significance in ANOVA tests run for each group; asterisks indicate pairwise significant differences tested post hoc), overall VGLUT1 densities were, in most animals, slightly depleted 1 wk after injury and very significantly depleted 6 wk and 6 mo after injury. B: group averages (n = 4 animals/group) indicated significant differences (ANOVA, P < 0.001). The control group was significantly different from all nerve injury groups (asterisks indicate P < 0.05, Holm-Sidak post hoc analysis). The 1 wk survival group (i.e., at the start of reinnervation) was less depleted than the 6 wk and 6 mo groups (times at which peripheral reinnervation has been completed). In contrast, differences between 6 wk and 6 mo were not significant. C: motoneurons labeled only with Fast Blue (FB) were not significantly different from dual-labeled motoneurons (confirmed MG reinnervating motoneurons) at 6 wk or 6 mo. D: as in A for individual animal averages in the 1 wk (medium gray bars), 4 (dark gray bars), and 6 mo (light gray bars) groups after MG nerve injury and reattachment [control uninjured group (black bars) is the same as in _A_]. In this case there was no significant interanimal variability within the 4 and 6 mo groups. E: comparison of MG motoneurons axotomized in the TN or in the MG nerve (MGN) and reinnervating the MG muscle (thus dual labeled) analyzed 6 mo after injury (n = 4 animals/group). VGLUT1 density depletions compared with control were similar in both groups. F: comparison between Fast Blue labeled only (FB) and dual-labeled MG motoneurons after MG nerve injury and reunion. No significant differences were detected at 4 mo, but a significant difference was found at 6 mo.
Fig. 6.
Synaptic remodeling on motoneurons after TN injuries. A–C: examples of NeuN-IR motoneurons (red, Cy3) surrounded by synaptic contacts (green, FITC) labeled with different synaptic markers [VGLUT2 (A), vesicular GABA/glycine transporter (VGAT, B), vesicular acetylcholine transporter (VAChT, C)] and imaged in the control side (A1, B1, C1) or in the experimental side 2 wk after injury with regeneration prevented (A2, B2, C2) or 12 wk after injury with regeneration allowed (A3, B3, C3). NeuN immunoreactivity decreases at 2 wk but recovers at 12 wk with muscle reinnervation. All 3 synaptic markers are decreased by axotomy at 2 wk after injury, but also all 3 show significant recovery at 12 wk after successful peripheral regeneration. Although bouton densities surrounding the cell bodies were greatly recovered, the synaptic puncta labeled by these vesicular markers frequently appeared smaller than in the control side. D: % depletions for each synaptic marker and comparisons to VGLUT1 at 2 or 4 wk after injury with regeneration prevented (black and light gray bars, respectively) and at 12 wk (dark gray bars) and 6 mo (white bars) after injury with regeneration allowed (and completed by this time). Asterisks indicate significant depletions in experimental side compared with control for each marker and date (*P < 0.05, **P < 0.01, ***P < 0.001, _t_-test). VGLUT1 contacts were very significantly depleted at all postinjury dates. VGLUT2 terminals recover but only after reinnervation. VGAT and VAChT terminals, in contrast, recover at 4 wk in the absence of peripheral regeneration, and recovery is not significantly different after regeneration in the periphery is completed.
Fig. 7.
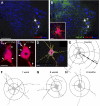
VGLUT1 contacts are diminished on the proximal dendrites of MG motoneurons after peripheral nerve injury. A and B: low-magnification confocal images of 2 control motoneurons retrogradely labeled with CTb-555 (white) from the MG muscle and located in a section through the caudal lumbar 5 counterstained with Nissl (blue, 640-Neurotrace). CTb-555 labeling is shown in white to best demonstrate the extent of dendritic labeling within the ventral horn. VGLUT1-IR puncta (green, FITC) are superimposed in B. Inset: 2-dimensional projection of all VGLUT1-IR contacts on the cell body and proximal dendrites of the motoneuron indicated with an asterisk in B. C1 and C2: 2 confocal planes (z depths indicated from the surface of the section) of the cell body and dendrites of the same motoneuron. Arrows indicate positions of VGLUT1 contacts. D: Neurolucida reconstruction of the same cell showing skeleton outlines of dendritic tracings and optical planes through the cell body. VGLUT1 contacts are plotted on the dendrites (circles) and cell body (triangles). The Neurolucida tracing is superimposed in 1 optical plane of the confocal image stack. E: Neurolucida cell reconstruction of the same control motoneuron showing dendritic thicknesses, the positions of dendritic VGLUT1 contacts (black circles, somatic contacts are not shown), and 50-μm Sholl bins used in the analyses. F–H: VGLUT1 contacts plotted on motoneuron reconstructions 1 wk (F), 6 wk (G), and 6 mo (H) after injury. The largest loss of VGLUT1 contacts on dendrites is observed proximally, particularly in the first Sholl bin. Scale bars: in A, 250 μm (B at the same magnification); in inset, C, and D, 30 μm.
Fig. 8.
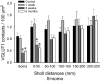
Quantitative analysis of VGLUT1 densities on the dendrites of MG motoneurons after peripheral nerve injury. Average VGLUT1 density was depleted on the soma and first and second 50-μm Sholl bins of motoneurons from 6 wk (dark gray bars) and 6 mo (white bars) animals compared with control (black bars) (n = 10 motoneurons analyzed in each group). However, these depletions were statistically significant only on the soma and most proximal dendritic bin (*P < 0.05, 1-way ANOVA followed by post hoc Bonferroni _t_-test comparisons of each experimental group with control). No significant depletions were observed 1 wk after injury at any location on the dendritic tree (light gray bars). Depletions in cell body and first 100 μm of dendrite represent ∼50% loss of VGLUT1 synapses, while in the dendritic arbor the overall loss was ∼25% because of the preservation (and even a nonsignificant slight increase) of VGLUT1 synapses at more distal locations.
Fig. 9.
Group I excitatory postsynaptic potentials (EPSPs) in motoneurons that regenerated peripherally after cut and reunion of the TN. A: example of a motoneuron antidromic action potential evoked by stimulation of the TN distal to the nerve injury. B: muscle twitch registered in the MG muscle by the same stimulus to the TN and demonstrating successful muscle reinnervation. C: superimposition of compound EPSPs recorded in all 20 motoneurons evoked by stimulation of the TN distal to the injury (arrow indicates stimulation artifact). Each EPSP trace is the average of 10–15 trials elicited at 2 Hz. All recorded motoneurons showed the presence of EPSPs, but their amplitude varied from cell to cell. Stimulation intensity was adjusted such that EPSPs were revealed without contamination from antidromic or orthodromic action potentials. Therefore it was not possible to measure the maximal amplitudes in response to the largest possible axon recruitment by the peripheral stimulus in the tibial nerve. However, 17 of the 20 EPSPs were smaller than 2 mV, while on average TN EPSPs measured in similar conditions are ∼5 mV in amplitude and almost always larger than 2 mV (Bichler et al. 2007).
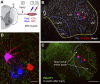
Fig. 10.
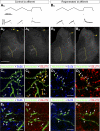
Trajectories and VGLUT immunoreactivities of IA afferent fibers in control animals or after peripheral nerve injury and regeneration. A1 and B1: responses of control (A1) and regenerated (B1) sensory afferents to triangular and ramp and hold muscle stretches. The muscle stretch stimulus is shown in the top traces, and the recorded responses depicted as firing frequency time plots are shown in the bottom traces. Both fibers faithfully encode muscle stretch parameters with dynamic responses typical of IA sensory afferents, similar levels of static response during hold phases, and similar history dependence of the initial burst in triangular stretches. Stretch responses were indistinguishable in control and regenerated afferents. A2 and A3: low-magnification epifluorescence images of 2 semiserial spinal cord sections containing labeled collaterals of the sensory axon with stretch responses shown in A1. Segments of the parent axons are visible in the dorsal columns (arrows) and collaterals with terminal branches in laminae V, VII, and IX are easily observed (border between laminae IX and VII is labeled with a dashed yellow line). The arborization in lamina IX was consistently very profuse in all control afferents. B2 and B3: images similar to A2 and A3 but for labeled collaterals from a regenerated axon. Note the parent axon in the dorsal column (arrows) and similar dorsomedial to ventrolateral trajectories of the central collaterals innervating the spinal cord, but these stop before entering lamina IX. C: high-magnification confocal microscopy of a varicose collateral from a neurobiotin-filled IA afferent (FITC-streptavidin, green) with several boutons (arrows) containing immunoreactivity for the synaptic protein SV2b (C1, blue, Cy5) and VGLUT1 (C2, red, Cy3). There was always a perfect correspondence between SV2b-containing varicosities and VGLUT1. D: similar high-magnification images of varicosities (arrows) from a regenerated afferent. All varicosities contained Sv2b (D1) and VGLUT1 (D2); however, neurobiotin-filled varicosities (FTIC, green in D1 and D2) appear of smaller size than in control uninjured afferents. E and F: similar sequence of images but for sections immunolabeled with SV2b and VGLUT2. SV2b-IR neurobiotin-filled varicosities (arrows) in control (E1) and regenerated (F1) afferents lack visible VGLUT2 immunoreactivity (E2 and F2). Scale bars, 500 μm in A1 (A2, B1, and B2 are at the same magnification); 10 μm in C1, D1, E1, and F1 (C2, D2, E2, and F2 are at the same magnification).
Fig. 11.
VGLUT1 immunoreactivity is retained in the central synaptic boutons of regenerated IA fibers, but the boutons become smaller. A: average immunofluorescence intensities for VGLUT1 and VGLUT2 in the central terminals of IA afferents recovered in control (gray bars, n = 335 boutons) and experimental (black bars, n = 331 boutons) animals. White dots indicate the average background fluorescence in all images (confocal microscope offsets were always 0). Background fluorescence was distributed very narrowly, as indicated by the lines indicating the upper and lower confidence limit intervals containing 99.9% of the distribution of background values. B: average normalized VGLUT1 (gray bars) and VGLUT2 (black bars) immunofluorescence intensity in each of the 4 control and experimental animals (the immunofluorescence of each varicosity was divided by the local background fluorescence calculated from 3 adjacent regions of equal size to the varicosity, see
methods
). No values for VGLUT2 were obtained in experimental animal 3 because in this animal the immunoreaction was abnormally weak and did not penetrate enough into the tissue. C: cumulative probability functions of the distributions of immunofluorescence densities for VGLUT1 and VGLUT2 normalized against background (bck). Kolmogorov-Smirnov tests showed no differences between the distribution of intensities in control (circles) and experimental (diamonds) values for VGLUT1 (gray plots) or VGLUT2 (black plots). D: maximum projection of the cross-sectional area of neurobiotin-labeled synaptic boutons (aka SV2b-IR) in the 4 control and 4 experimental animals. The average cross-sectional areas of all IA afferent synaptic boutons in experimental animals were significantly smaller than any of the control animals (P < 0.001, ANOVA on ranks, followed by post hoc Dunn's test pairwise comparisons, *P < 0.05 in all cases). E: probability distributions of bouton sizes in each individual animal (dotted lines) and average distributions for control and experimental groups shown in thicker line plots (gray, control; black, experimental). Bouton sizes show a significant skew toward relatively large boutons in the control distribution. In regenerated afferents most of the boutons are small and many of the larger boutons are not present. Thus the bouton population in regenerated afferents does not scale back in size proportionally from control IA boutons, but rather regenerated afferents generally lack large boutons, increasing the probability of smaller boutons in the population. F: cross-sectional bouton areas in the 4 control and 4 experimental animals distributed by laminar location. N.D. indicates that no boutons were detected in lamina IX from any of the regenerated IA afferents. In 1 experimental animal no boutons were detected in lamina VII, either. Remaining boutons in laminae V and VII were on average smaller in the 4 experimental animals compared with the control populations (P < 0.001, ANOVA on ranks, followed by post hoc Dunn's test pairwise comparisons, *P < 0.05 in all cases). Error bars indicate SE in all histograms.
Similar articles
- Synaptic Plasticity on Motoneurons After Axotomy: A Necessary Change in Paradigm.
Alvarez FJ, Rotterman TM, Akhter ET, Lane AR, English AW, Cope TC. Alvarez FJ, et al. Front Mol Neurosci. 2020 Apr 30;13:68. doi: 10.3389/fnmol.2020.00068. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32425754 Free PMC article. Review. - Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. II. Loss of functional connectivity with motoneurons.
Bullinger KL, Nardelli P, Pinter MJ, Alvarez FJ, Cope TC. Bullinger KL, et al. J Neurophysiol. 2011 Nov;106(5):2471-85. doi: 10.1152/jn.01097.2010. Epub 2011 Aug 10. J Neurophysiol. 2011. PMID: 21832030 Free PMC article. - Normal distribution of VGLUT1 synapses on spinal motoneuron dendrites and their reorganization after nerve injury.
Rotterman TM, Nardelli P, Cope TC, Alvarez FJ. Rotterman TM, et al. J Neurosci. 2014 Mar 5;34(10):3475-92. doi: 10.1523/JNEUROSCI.4768-13.2014. J Neurosci. 2014. PMID: 24599449 Free PMC article. - VGLUT1 synapses and P-boutons on regenerating motoneurons after nerve crush.
Schultz AJ, Rotterman TM, Dwarakanath A, Alvarez FJ. Schultz AJ, et al. J Comp Neurol. 2017 Sep 1;525(13):2876-2889. doi: 10.1002/cne.24244. Epub 2017 Jun 15. J Comp Neurol. 2017. PMID: 28543879 Free PMC article. - Applications of Proteomics to Nerve Regeneration Research.
Massing MW, Robinson GA, Marx CE, Alzate O, Madison RD. Massing MW, et al. In: Alzate O, editor. Neuroproteomics. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 15. In: Alzate O, editor. Neuroproteomics. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 15. PMID: 21882439 Free Books & Documents. Review.
Cited by
- Motoneuron deafferentation and gliosis occur in association with neuromuscular regressive changes during ageing in mice.
Blasco A, Gras S, Mòdol-Caballero G, Tarabal O, Casanovas A, Piedrafita L, Barranco A, Das T, Pereira SL, Navarro X, Rueda R, Esquerda JE, Calderó J. Blasco A, et al. J Cachexia Sarcopenia Muscle. 2020 Dec;11(6):1628-1660. doi: 10.1002/jcsm.12599. Epub 2020 Jul 20. J Cachexia Sarcopenia Muscle. 2020. PMID: 32691534 Free PMC article. - Self-reinnervated muscles lose autogenic length feedback, but intermuscular feedback can recover functional connectivity.
Lyle MA, Prilutsky BI, Gregor RJ, Abelew TA, Nichols TR. Lyle MA, et al. J Neurophysiol. 2016 Sep 1;116(3):1055-67. doi: 10.1152/jn.00335.2016. Epub 2016 Jun 15. J Neurophysiol. 2016. PMID: 27306676 Free PMC article. - Synaptic Plasticity on Motoneurons After Axotomy: A Necessary Change in Paradigm.
Alvarez FJ, Rotterman TM, Akhter ET, Lane AR, English AW, Cope TC. Alvarez FJ, et al. Front Mol Neurosci. 2020 Apr 30;13:68. doi: 10.3389/fnmol.2020.00068. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32425754 Free PMC article. Review. - Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. II. Loss of functional connectivity with motoneurons.
Bullinger KL, Nardelli P, Pinter MJ, Alvarez FJ, Cope TC. Bullinger KL, et al. J Neurophysiol. 2011 Nov;106(5):2471-85. doi: 10.1152/jn.01097.2010. Epub 2011 Aug 10. J Neurophysiol. 2011. PMID: 21832030 Free PMC article.
References
- Abelew TA, Miller MD, Cope TC, Nichols TR. Local loss of proprioception results in disruption of interjoint coordination during locomotion in the cat. J Neurophysiol 84: 2709–2714, 2000 - PubMed
- Alstermark B, Ogawa J, Isa T. Lack of monosynaptic corticomotoneuronal EPSPs in rats: disynaptic EPSPs mediated via reticulospinal neurons and polysynaptic EPSPs via segmental interneurons. J Neurophysiol 91: 1832–1839, 2004 - PubMed
- Alvarez FJ, Nardelli P, Bullinger KL, Ukpabi N, Crum JM, Zerda R, Kraszpulski M, Cope TC. VGLUT1 content in central synapses of normal and regenerated Ia afferents (Abstract). 2008 Neuroscience Meeting Planner Washington, DC: Society for Neuroscience, 2008, Program No. 74.1 (online)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical