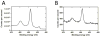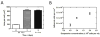Use of autologous blood-derived endothelial progenitor cells at point-of-care to protect against implant thrombosis in a large animal model - PubMed (original) (raw)
Use of autologous blood-derived endothelial progenitor cells at point-of-care to protect against implant thrombosis in a large animal model
Alexandra E Jantzen et al. Biomaterials. 2011 Nov.
Abstract
Titanium (Ti) is commonly utilized in many cardiovascular devices, e.g. as a component of Nitinol stents, intra- and extracorporeal mechanical circulatory assist devices, but is associated with the risk of thromboemboli formation. We propose to solve this problem by lining the Ti blood-contacting surfaces with autologous peripheral blood-derived late outgrowth endothelial progenitor cells (EPCs) after having previously demonstrated that these EPCs adhere to and grow on Ti under physiological shear stresses and functionally adapt to their environment under flow conditions ex vivo. Autologous fluorescently-labeled porcine EPCs were seeded at the point-of-care in the operating room onto Ti tubes for 30 min and implanted into the pro-thrombotic environment of the inferior vena cava of swine (n = 8). After 3 days, Ti tubes were explanted, disassembled, and the blood-contacting surface was imaged. A blinded analysis found all 4 cell-seeded implants to be free of clot, whereas 4 controls without EPCs were either entirely occluded or partially thrombosed. Pre-labeled EPCs had spread and were present on all 4 cell-seeded implants while no endothelial cells were observed on control implants. These results suggest that late outgrowth autologous EPCs represent a promising source of lining Ti implants to reduce thrombosis in vivo.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
Fig. 1
(A) Ti tube seeding device and Ti tube seeding chamber. The Ti tube is placed inside the aluminum holder. To assemble the seeding chamber, a ‘cut-off’ syringe head with luer is attached via silicone tubing to the Ti tube (left end of Ti tube) and a 5 cc syringe attached via silicone tubing (right end of Ti tube). The level gauge ensures equal distribution of EPCs during rotation of the seeding chamber. A sterile sheath surrounding the seeding chamber has been removed in order to facilitate viewing of the seeding chamber inside the aluminum holder. (B) Intraoperative view of skeletonized infrarenal IVC, prior to venotomy. The proximal end of the IVC is encircled with a yellow vessel loop (left). The overlying right renal artery is encircled with a blue vessel loop (left). A blue vessel loop encircles the left 7th lumbar vein. The distal IVC is encircled with a yellow vessel loop at its bifurcation into the common iliac veins (right) and the right external iliac artery is encircled with an orange vessel loop as it crosses the right common iliac vein (far right). (C) Ti tube insertion into IVC. Both ends of the IVC are clamped. The blue PVC heat shrink tubing is recognizable inside the IVC. (D) Post-insertion view of the infrarenal IVC. The yellow vessel loop encircles the proximal IVC. Note the 6-0 polypropylene ‘stay suture’ that was placed through the adventitia and PVC heat shrink tubing to prevent the device from migrating (white arrow).
Fig. 2
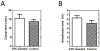
(A) Intraoperative clamp times of IVC during device insertion. Clamp times were not significantly different between EPC-seeded and control implant groups (15.5 ± 2.3 min and 13.8 ± 1.2 min, respectively, p = 0.517, n = 4 for each group, two-tailed t-test). (B) Total time of anesthesia during surgery (in hours). Duration of anesthesia was not significantly different between EPC-seeded and control implant groups (6 hours 19 minutes ± 31 minutes vs. 5 hours 7 minutes ± 41 minutes, p = 0.21, n = 4 for each group, two-tailed t-test).
Fig. 3
(A) XPS spectrum of Ti tube with binding energy peaking at 458.9 eV, corresponding to the Ti 2p3/2 electron configuration. (B) XPS spectrum of the Nitinol stent with binding energy peaking at 459.1 eV, indicating comparable TiO2 composition. Note that the Nitinol stent was imaged in its expanded state with a small field of view focusing on a stent strut only, whereas the titanium tube was imaged with a larger field of view, which is reflected by the difference in signal intensity (measured in counts per second) between the Ti tube and Nitinol stent.
Fig. 4
(A) EPC Spreading on Ti tubes at time zero after seeding, after 1 day of static culture ex vivo, and after three days in vivo. EPCs’ area was significantly greater at 1 and 3 days than at time zero (p < 0.0001, n = 3 at 1 day, n = 4 at 0 and 3 days, 1-way ANOVA and post hoc t-test). (B) EPC surface coverage with suspension density. The number of adherent cells per area significantly increased as suspension concentration increased (p < 0.001, r2 = 0.82, n = 3 for each concentration, linear regression analysis).
Fig. 5
Categorization of thrombosis outcomes. Representative pictures of Ti tubes (A, B) fully clotted, (C, D) partially clotted, and (E, F) not clotted. Tubes are shown in two orientations - a transverse view with tubes intact (left column) - and in a longitudinal view of the inner surface after tube dissection (right column).
Fig. 6
EPCs on Ti surface were stained for PECAM-1 (green) after 3 days in porcine IVC. (A) Scale bar = 200 μm. (B) EPCs additionally showing pre-implantation stain PKH26 (red) and nuclei stained with Hoechst 34580 (blue). Scale bar = 50 μm. (C) Cell nuclei on bare metal control implant surface stained with Hoechst 34580, showing presence of macrophages, T-cells and granulocytes in a non-uniform distribution. Scale bar = 100 μm.
Similar articles
- Autologous endothelial progenitor cell-seeding technology and biocompatibility testing for cardiovascular devices in large animal model.
Jantzen AE, Lane WO, Gage SM, Haseltine JM, Galinat LJ, Jamiolkowski RM, Lin FH, Truskey GA, Achneck HE. Jantzen AE, et al. J Vis Exp. 2011 Sep 9;(55):3197. doi: 10.3791/3197. J Vis Exp. 2011. PMID: 21931293 Free PMC article. - The biocompatibility of titanium cardiovascular devices seeded with autologous blood-derived endothelial progenitor cells: EPC-seeded antithrombotic Ti implants.
Achneck HE, Jamiolkowski RM, Jantzen AE, Haseltine JM, Lane WO, Huang JK, Galinat LJ, Serpe MJ, Lin FH, Li M, Parikh A, Ma L, Chen T, Sileshi B, Milano CA, Wallace CS, Stabler TV, Allen JD, Truskey GA, Lawson JH. Achneck HE, et al. Biomaterials. 2011 Jan;32(1):10-8. doi: 10.1016/j.biomaterials.2010.08.073. Epub 2010 Nov 5. Biomaterials. 2011. PMID: 20926131 Free PMC article. - [Preliminary in vivo evaluation of tissue engineered venous grafts fabricated based on endothelial progenitor cells].
Wu YF, Zhang J, Gu YQ, Li JX, Chen XS, Chen L, Chen B, Guo LR, Luo T, Liao CJ, Wu X, Yu HX, Wang ZG. Wu YF, et al. Zhonghua Wai Ke Za Zhi. 2007 Apr 1;45(7):491-5. Zhonghua Wai Ke Za Zhi. 2007. PMID: 17686312 Chinese. - Strategies for Improving Endothelial Cell Adhesion to Blood-Contacting Medical Devices.
Wolfe JT, Shradhanjali A, Tefft BJ. Wolfe JT, et al. Tissue Eng Part B Rev. 2022 Oct;28(5):1067-1092. doi: 10.1089/ten.TEB.2021.0148. Epub 2022 Feb 7. Tissue Eng Part B Rev. 2022. PMID: 34693761 Review. - [Endothelial progenitor cells: the new target of anti-atherosclerosis drugs].
Zhang B, Niu P, Li H, Jia S. Zhang B, et al. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2013 Mar;38(3):307-12. doi: 10.3969/j.issn.1672-7347.2013.03.015. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2013. PMID: 23545827 Review. Chinese.
Cited by
- Development of In Vitro Endothelialised Stents - Review.
Tsukada J, Mela P, Jinzaki M, Tsukada H, Schmitz-Rode T, Vogt F. Tsukada J, et al. Stem Cell Rev Rep. 2022 Jan;18(1):179-197. doi: 10.1007/s12015-021-10238-3. Epub 2021 Aug 17. Stem Cell Rev Rep. 2022. PMID: 34403073 Review. - Efficient and safe correction of hemophilia A by lentiviral vector-transduced BOECs in an implantable device.
Olgasi C, Borsotti C, Merlin S, Bergmann T, Bittorf P, Adewoye AB, Wragg N, Patterson K, Calabria A, Benedicenti F, Cucci A, Borchiellini A, Pollio B, Montini E, Mazzuca DM, Zierau M, Stolzing A, Toleikis PM, Braspenning J, Follenzi A. Olgasi C, et al. Mol Ther Methods Clin Dev. 2021 Nov 3;23:551-566. doi: 10.1016/j.omtm.2021.10.015. eCollection 2021 Dec 10. Mol Ther Methods Clin Dev. 2021. PMID: 34853801 Free PMC article. - Nanoparticle-Mediated Cell Capture Enables Rapid Endothelialization of a Novel Bare Metal Stent.
Tefft BJ, Uthamaraj S, Harbuzariu A, Harburn JJ, Witt TA, Newman B, Psaltis PJ, Hlinomaz O, Holmes DR Jr, Gulati R, Simari RD, Dragomir-Daescu D, Sandhu GS. Tefft BJ, et al. Tissue Eng Part A. 2018 Jul;24(13-14):1157-1166. doi: 10.1089/ten.TEA.2017.0404. Epub 2018 Mar 13. Tissue Eng Part A. 2018. PMID: 29431053 Free PMC article. - Continuously Grooved Stent Struts for Enhanced Endothelial Cell Seeding.
Ter Meer M, Daamen WF, Hoogeveen YL, van Son GJF, Schaffer JE, van der Vliet JA, Kool LJS, van den Heuvel LP. Ter Meer M, et al. Cardiovasc Intervent Radiol. 2017 Aug;40(8):1237-1245. doi: 10.1007/s00270-017-1659-4. Epub 2017 May 3. Cardiovasc Intervent Radiol. 2017. PMID: 28470391 Free PMC article. - MiR-9 promotes angiogenesis of endothelial progenitor cell to facilitate thrombi recanalization via targeting TRPM7 through PI3K/Akt/autophagy pathway.
Zhou DM, Sun LL, Zhu J, Chen B, Li XQ, Li WD. Zhou DM, et al. J Cell Mol Med. 2020 Apr;24(8):4624-4632. doi: 10.1111/jcmm.15124. Epub 2020 Mar 9. J Cell Mol Med. 2020. PMID: 32147957 Free PMC article.
References
- Achneck HE, Sileshi B, Parikh A, Milano CA, Welsby IJ, Lawson JH. Pathophysiology of bleeding and clotting in the cardiac surgery patient: from vascular endothelium to circulatory assist device surface. Circulation. 2010;122:2068–77. - PubMed
- Schillinger M, Sabeti S, Dick P, Amighi J, Mlekusch W, Schlager O, et al. Sustained benefit at 2 years of primary femoropopliteal stenting compared with balloon angioplasty with optional stenting. Circulation. 2007;115:2745–9. - PubMed
- Maleux G, Marrannes J, Heye S, Daenens K, Verhamme P, Thijs V. Outcome of carotid artery stenting at 2 years follow-up: comparison of nitinol open cell versus stainless steel closed cell stent design. J Cardiovasc Surg (Torino) 2009;50:669–75. - PubMed
- Dick P, Wallner H, Sabeti S, Loewe C, Mlekusch W, Lammer J, et al. Balloon angioplasty versus stenting with nitinol stents in intermediate length superficial femoral artery lesions. Catheter Cardiovasc Interv. 2009;74:1090–5. - PubMed
- Duda SH, Bosiers M, Lammer J, Scheinert D, Zeller T, Oliva V, et al. Drug-eluting and bare nitinol stents for the treatment of atherosclerotic lesions in the superficial femoral artery: long-term results from the SIROCCO trial. J Endovasc Ther. 2006;13:701–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 HL109897-01/HL/NHLBI NIH HHS/United States
- RC1 HL099863-01/HL/NHLBI NIH HHS/United States
- RC1HL099863-01/HL/NHLBI NIH HHS/United States
- RC1 HL099863/HL/NHLBI NIH HHS/United States
- R21 HL109897/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical