β2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system - PubMed (original) (raw)
β2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system
Gergely Vida et al. FASEB J. 2011 Dec.
Abstract
The nervous system is classically organized into sympathetic and parasympathetic systems acting in opposition to maintain physiological homeostasis. Here, we report that both systems converge in the activation of β2-adrenoceptors of splenic regulatory lymphocytes to control systemic inflammation. Vagus nerve stimulation fails to control serum TNF levels in either β2-knockout or lymphocyte-deficient nude mice. Unlike typical suppressor CD25(+) cells, the transfer of CD4(+)CD25(-) regulatory lymphocytes reestablishes the anti-inflammatory potential of the vagus nerve and β2-agonists to control inflammation in both β2-knockout and nude mice. β2-Agonists inhibit cytokine production in splenocytes (IC(50)≈ 1 μM) and prevent systemic inflammation in wild-type but not in β2-knockout mice. β2-Agonists rescue wild-type mice from established polymicrobial peritonitis in a clinically relevant time frame. Regulatory lymphocytes reestablish the anti-inflammatory potential of β2-agonists to control systemic inflammation, organ damage, and lethal endotoxic shock in β2-knockout mice. These results indicate that β2-adrenoceptors in regulatory lymphocytes are critical for the anti-inflammatory potential of the parasympathetic vagus nerve, and they represent a potential pharmacological target for sepsis.
Figures
Figure 1.
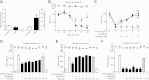
Norepinephrine inhibits cytokine production in splenocytes via the β2-adrenoceptors. A) Adult male mice underwent sham surgery (control) or VNS. Epinephrine (E) and norepinephrine (NE) plasma levels were analyzed by ELISA at 15 min after stimulation. *P < 0.01 vs. control; Mann-Whitney U test (_n_=4/group). B) Primary cultures of splenocytes were treated with LPS (100 ng/ml) and E or NE. C) Primary cultures of splenocytes were treated with β-blockers, propranolol (PR) and nadolol (NA), or α-blocker, phentolamine (PH), 30 min prior to LPS and NE. D–F) Primary cultures of splenocytes were treated with β1-blocker, atenolol (AT; D), β2-blocker, butoxamine (BU; E), or β3-blocker, SR59230A (SR; F). In all the experiments, blockers were administered 30 min prior to LPS and NE, and TNF concentrations were analyzed in the conditioned medium at 3 h after treatment. Top panels represent cell survival as determined by MTT assay. *P < 0.01; 1-way ANOVA with Bonferroni's corrections (_n_=4).
Figure 2.
The vagus nerve inhibits systemic inflammation via β2-adrenoceptors. Adult male mice were treated with reserpine (5 mg/kg i.p.; A); fusaric acid (40 mg/kg i.p.; B); β-blocker, nadolol (NA; 15 mg/kg i.p.; C); β1-blocker, atenolol (AT; 15 mg/kg i.p.; D); β2-blocker, butoxamine (BU; 15 mg/kg i.p.; E); or β3-blocker, SR59230A (SR; 15 mg/kg i.p.; F), 30 min prior to VNS or sham surgery. Sham surgery or VNS was performed during 10 min before the LPS challenge. *P < 0.01 vs. LPS; 1-way ANOVA with Bonferroni's corrections (_n_=4).
Figure 3.
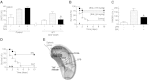
Wild-type CD3+CD4+CD25− lymphocytes reestablish neuromodulation in β2-knockout mice. A) Primary cultures of splenocytes (SPLN) from wild-type (WT) or β2-knockout (β2KO) mice were treated with norepinephrine (NE), 30 min before LPS challenge (100 ng/ml). Top panels represent cell survival as determined by MTT assay. B) WT or β2KO mice underwent sham surgery or VNS during 10 min before endotoxemia (LPS; 6 mg/kg i.p). Serum TNF levels were analyzed by ELISA at 90 min after LPS. C) WT or β2KO mice underwent sham surgery or VNS, and plasma levels of NE were analyzed at 15 min after stimulation. *P < 0.01 vs. control; Mann-Whitney U test (_n_=4/group). D) Primary SPLN cultures from WT or lymphocyte-deficient nude (Nude) mice were treated with NE, 30 min before LPS challenge (100 ng/ml). TNF concentrations were analyzed in the conditioned medium at 3 h after the LPS stimulation. Top panels represent cell survival as determined by MTT assay. E, F) Lymphocyte-deficient nude mice (E) or β2KO mice (F) received vehicle (control), classical suppressor CD3+CD4+CD25+, or CD3+CD4+CD25− splenocytes from WT or β2KO mice. Animals underwent sham surgery or VNS during 10 min before endotoxemia. Serum TNF levels were analyzed by ELISA at 90 min post-LPS. *P < 0.01 vs. LPS; 1-way ANOVA with Bonferroni's corrections (_n_=4).
Figure 4.
Wild-type CD3+CD4+CD25− lymphocytes reestablish the hemodynamics and anti-inflammatory potential of β2-agonist in β2-knockout mice. A) Adult male wild-type mice were treated with specific β2-agonists terbutaline (TER) or salbutamol (SAL), 30 min before the LPS challenge (LPS; 6 mg/kg i.p.). B) Primary culture of splenocytes from wild-type (WT) or β2-knockout (β2KO) mice were treated with SAL, 30 min before LPS challenge (100 ng/ml). TNF concentration was analyzed by ELISA in the conditioned medium at 3 h poststimulation. C) Adult male WT or β2KO mice were treated with vehicle (control), CD3+CD4+CD25− or CD3+CD4+CD25+ lymphocytes from WT mice. Mice were treated with β2-agonist SAL (15 mg/kg i.p.), 30 min prior the endotoxemia. Serum TNF levels were analyzed by ELISA at 90 min after LPS. *P < 0.01 vs. LPS; 1-way ANOVA with Bonferroni's corrections (_n_=4). D, E) Adult β2KO mice received vehicle (control) or CD3+CD4+CD25− (CD25−) lymphocytes from WT mice, and then treated with β2-agonist SAL (15 mg/kg i.p.). Mean arterial blood pressure (MABP; D) and heart rate (HR; E) were recorded.
Figure 5.
β2-Agonist prevents organ damage and rescues mice from established polymicrobial peritonitis. A) Adult β2-knockout (β2KO) mice were given vehicle (control), or CD3+CD4+CD25− lymphocytes from wild-type (WT) mice with vehicle or β2-agonist salbutamol (SAL; 15 mg/kg i.p.). Serum aspartate transaminase (AST) levels were analyzed at 90 min after LPS. *P < 0.01 vs. LPS; 1-way ANOVA with Bonferroni's corrections (_n_=4). B) Adult male mice were treated with β2-agonist SAL. Survival was represented in a Kaplan-Meier analysis (_n_=20/group; P<0.01, survival log-rank test vs. control). Arrow represents the single dose of vehicle (control) or SAL (6 or 15 mg/kg i.p.) given 24 h after LPS challenge. C) Serum HMGB1 levels were analyzed by Western blot at 44 h after LPS challenge. Graph represents densitometric units (d.u.) from scanning of the film. *P < 0.01 vs. LPS; 1-way ANOVA with Bonferroni's corrections (_n_=5/group). D) Arrows represent 3 doses of vehicle (control) or SAL (15 mg/kg i.p.) started 24 h after CLP, and given every 12 h. Survival was analyzed and represented in a Kaplan-Meier analysis (_n_=15/group; P<0.01, survival log-rank test vs. control). E) VNS leads to the activation of the splenic nerve and subsequent release of norepinephrine (NE) in the spleen. Both NE (splenic nerve) and β2AR-agonists (salbutamol) inhibit cytokine production in the spleen and systemic inflammation in experimental sepsis through a mechanism specifically mediated by the β2-adrenoceptors of CD3+CD4+CD25− lymphocytes. This cellular mechanism resembles the innervation of the periarterial lymphoid sheath (PALS), where the splenic nerve makes synaptic-like connections with T lymphocytes located in the vicinity of macrophages (37).
Similar articles
- Cholinergic regulatory lymphocytes re-establish neuromodulation of innate immune responses in sepsis.
Peña G, Cai B, Ramos L, Vida G, Deitch EA, Ulloa L. Peña G, et al. J Immunol. 2011 Jul 15;187(2):718-25. doi: 10.4049/jimmunol.1100013. Epub 2011 Jun 10. J Immunol. 2011. PMID: 21666060 Free PMC article. - α7-cholinergic receptor mediates vagal induction of splenic norepinephrine.
Vida G, Peña G, Deitch EA, Ulloa L. Vida G, et al. J Immunol. 2011 Apr 1;186(7):4340-6. doi: 10.4049/jimmunol.1003722. Epub 2011 Feb 21. J Immunol. 2011. PMID: 21339364 Free PMC article. - Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis.
Huston JM, Gallowitsch-Puerta M, Ochani M, Ochani K, Yuan R, Rosas-Ballina M, Ashok M, Goldstein RS, Chavan S, Pavlov VA, Metz CN, Yang H, Czura CJ, Wang H, Tracey KJ. Huston JM, et al. Crit Care Med. 2007 Dec;35(12):2762-8. doi: 10.1097/01.CCM.0000288102.15975.BA. Crit Care Med. 2007. PMID: 17901837 - The vagus nerve and the inflammatory reflex: wandering on a new treatment paradigm for systemic inflammation and sepsis.
Huston JM. Huston JM. Surg Infect (Larchmt). 2012 Aug;13(4):187-93. doi: 10.1089/sur.2012.126. Epub 2012 Aug 22. Surg Infect (Larchmt). 2012. PMID: 22913335 Review. - The cholinergic anti-inflammatory pathway: a critical review.
Martelli D, McKinley MJ, McAllen RM. Martelli D, et al. Auton Neurosci. 2014 May;182:65-9. doi: 10.1016/j.autneu.2013.12.007. Epub 2013 Dec 24. Auton Neurosci. 2014. PMID: 24411268 Review.
Cited by
- Neural regulation of hematopoiesis, inflammation, and cancer.
Hanoun M, Maryanovich M, Arnal-Estapé A, Frenette PS. Hanoun M, et al. Neuron. 2015 Apr 22;86(2):360-73. doi: 10.1016/j.neuron.2015.01.026. Neuron. 2015. PMID: 25905810 Free PMC article. Review. - Proposed toxic and hypoxic impairment of a brainstem locus in autism.
McGinnis WR, Audhya T, Edelson SM. McGinnis WR, et al. Int J Environ Res Public Health. 2013 Dec 11;10(12):6955-7000. doi: 10.3390/ijerph10126955. Int J Environ Res Public Health. 2013. PMID: 24336025 Free PMC article. - Splenic SUMO1 controls systemic inflammation in experimental sepsis.
Youssef A, Mohammed BK, Prasad A, Del Aguila A, Bassi G, Yang W, Ulloa L. Youssef A, et al. Front Immunol. 2023 Jul 13;14:1200939. doi: 10.3389/fimmu.2023.1200939. eCollection 2023. Front Immunol. 2023. PMID: 37520526 Free PMC article. - Age-Related Variation in Sympathetic Nerve Distribution in the Human Spleen.
Cleypool CGJ, Brinkman DJ, Mackaaij C, Nikkels PGJ, Nolte MA, Luyer MD, de Jonge WJ, Bleys RLAW. Cleypool CGJ, et al. Front Neurosci. 2021 Oct 14;15:726825. doi: 10.3389/fnins.2021.726825. eCollection 2021. Front Neurosci. 2021. PMID: 34720859 Free PMC article. - Neuroendocrine regulation of inflammation.
Padro CJ, Sanders VM. Padro CJ, et al. Semin Immunol. 2014 Oct;26(5):357-68. doi: 10.1016/j.smim.2014.01.003. Epub 2014 Jan 31. Semin Immunol. 2014. PMID: 24486056 Free PMC article. Review.
References
- Nathan C. (2002) Points of control in inflammation. Nature 420, 846–852 - PubMed
- Ulloa L. (2005) The vagus nerve and the nicotinic anti-inflammatory pathway. Nat. Rev. 4, 673–684 - PubMed
- Ulloa L., Tracey K. J. (2005) The “cytokine profile”: a code for sepsis. Trends Mol. Med. 11, 56–63 - PubMed
- Sands K. E., Bates D. W., Lanken P. N., Graman P. S., Hibberd P. L., Kahn K. L., Parsonnet J., Panzer R., Orav E. J., Snydman D. R. (1997) Epidemiology of sepsis syndrome in 8 academic medical centers. Academic Medical Center Consortium Sepsis Project Working Group. JAMA 278, 234–240 - PubMed
- Angus D. C. (2007) Caring for the critically ill patient: challenges and opportunities. JAMA 298, 456–458 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials