Passive immunization with anti-Tau antibodies in two transgenic models: reduction of Tau pathology and delay of disease progression - PubMed (original) (raw)
. 2011 Sep 30;286(39):34457-67.
doi: 10.1074/jbc.M111.229633. Epub 2011 Aug 12.
Su Wu, Tracey K Murray, Robert Kinley, Claire V Cella, Helen Sims, Nicola Buckner, Jenna Hanmer, Peter Davies, Michael J O'Neill, Michael L Hutton, Martin Citron
Affiliations
- PMID: 21841002
- PMCID: PMC3190817
- DOI: 10.1074/jbc.M111.229633
Passive immunization with anti-Tau antibodies in two transgenic models: reduction of Tau pathology and delay of disease progression
Xiyun Chai et al. J Biol Chem. 2011.
Abstract
The microtubule-associated protein Tau plays a critical role in the pathogenesis of Alzheimer disease and several related disorders (tauopathies). In the disease Tau aggregates and becomes hyperphosphorylated forming paired helical and straight filaments, which can further condense into higher order neurofibrillary tangles in neurons. The development of this pathology is consistently associated with progressive neuronal loss and cognitive decline. The identification of tractable therapeutic targets in this pathway has been challenging, and consequently very few clinical studies addressing Tau pathology are underway. Recent active immunization studies have raised the possibility of modulating Tau pathology by activating the immune system. Here we report for the first time on passive immunotherapy for Tau in two well established transgenic models of Tau pathogenesis. We show that peripheral administration of two antibodies against pathological Tau forms significantly reduces biochemical Tau pathology in the JNPL3 mouse model. We further demonstrate that peripheral administration of the same antibodies in the more rapidly progressive P301S tauopathy model not only reduces Tau pathology quantitated by biochemical assays and immunohistochemistry, but also significantly delays the onset of motor function decline and weight loss. This is accompanied by a reduction in neurospheroids, providing direct evidence of reduced neurodegeneration. Thus, passive immunotherapy is effective at preventing the buildup of intracellular Tau pathology, neurospheroids, and associated symptoms, although the exact mechanism remains uncertain. Tau immunotherapy should therefore be considered as a therapeutic approach for the treatment of Alzheimer disease and other tauopathies.
Figures
FIGURE 1.
Procedure for preparation of tissue extracts. Most of our analyses are focused on the P1 fraction, which can be further processed by Sarkosyl extraction (11).
FIGURE 2.
Correlation of the AT8 signal in the P1 fraction with the AT8 signal in the Sarkosyl-insoluble fraction. Four JNPL3 mouse brain samples with different degrees of Tau pathology were processed to generate the P1 fraction, and then these P1 samples were subjected to Sarkosyl extraction (11) (see Fig. 1). Both the P1 samples and the Sarkosyl-extracted samples were subjected to AT8 ELISA, so that for each brain there is a P1 ELISA read (x axis) and then a second read after Sarkosyl ELISA extraction (y axis). Whereas the absolute AT8 signal is somewhat reduced after Sarkosyl extraction, the correlation is almost perfect, indicating that our P1 preparation is adequate.
FIGURE 3.
Correlation of the 64-kDa P1 Tau species on Western blots with neurofibrillary tangles (as detected by immunohistochemistry with antibody PG5).
FIGURE 4.
Biochemically detectable Tau pathology in JNPL3 mice is reduced after passive immunization with anti-Tau antibodies. A, AT8 Western blot of the P1 brain fraction from 6-month-old JNPL3 mice shows the specificity of the antibody for the 64-kDa band of hyperphosphorylated human Tau that comigrates with PHF Tau from AD brain (15) and correlates with functional deficits in several models of tauopathy (16). The 64-kDa band is weaker in the MC1 and PHF1 treatment groups compared with control antibody. B, HT7 Western blot of total Tau in total extracts shows no apparent differences between treatment groups.
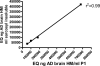
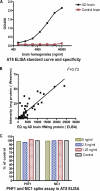
FIGURE 5.
AT8 ELISA. A, standard curve using AD and control brain homogenates. The normal Tau present in control brain does not lead to a detectable signal. B, AT8 Western blot and ELISA results in the P1 fraction of all mice in the study show a strong correlation (_r_2 = 0.73) between the two measures. C, spiking experiment. Addition of PHF1 and MC1 at the indicated concentrations to JNPL3 P1 extracts does not interfere with signal detection by the AT8 ELISA, demonstrating that AT8 signal changes in brains of mice treated with these antibodies are not ELISA artifacts.
FIGURE 6.
A, AT8 ELISA analysis of the JNPL3 mouse P1 brain fraction shows strong reduction in AT8 signal in both antibody treatment groups compared with control antibody. B, statistical analysis of the log-transformed AT8 ELISA data shows that the reduction is highly significant for both PHF1 and MC1.
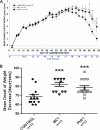
FIGURE 7.
Attenuation of body weight loss (A) and delay in onset of weight loss (B) after treatment with MC1 and PHF1. A, repeated-measures ANOVA indicated that there was a significant Day × Group interaction F(50,900) = 3.29, p < 0.0001 with both MC1 and PHF1 treatment reducing the weight loss from day 85 onward. Univariate planned comparisons revealed significant group effect from day 85 onward, p < 0.03. Post hoc Dunnett test compared with control antibody (Ab)-treated group: Day 85: F(2,36) = 4.07, p = 0.03, MC1 p = 0.01, PHF1 p = 0.03; Day 88: F(2,36) = 3.75, p = 0.03, MC1 p = 0.01, PHF1 p = 0.04; Day 93: F(2,36) = 4.00, p = 0.03, MC1 p = 0.009, PHF1 p = 0.05. In all cases; *, p < 0.05 versus control Ab-treated. Data are based on n = 13 mice per group. B, illustrates the mean day of onset of body weight loss in P301S mice. Results indicated that both MC1 and PHF1 treatment delayed the onset of weight loss. One-way ANOVA F(2,36) = 9.33, p < 0.001. Post hoc Dunnett test compared with control Ab-treated group; ***, p < 0.005. Data are based on 13 mice per group.
FIGURE 8.
Motor performance as measured by fall latency on Rota-Rod 1 week before sacrifice is improved by both PHF1 and MC1 versus control antibody treatment. Univariate planned comparisons revealed significant difference between control Ab-treated (n = 13) and Combined data (n = 26) compared with control Ab-treated group; *, p < 0.05; **, p < 0.01. For individual PHF1 and MC1 data there were significant improvements over control Ab-treated at several speeds using post hoc Dunnett's t test; *, p < 0.05; **, p < 0.01.
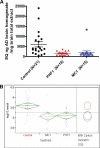
FIGURE 9.
Biochemical analysis of P301S extracts. A, HT7 Western blot of total Tau in total extracts shows no apparent differences between treatment groups. B–D, AT8 ELISA of P301S mice. All plots show AT8 signal (micrograms of AD brain homogenate that would produce the same ELISA signal) per milligram of mouse tissue homogenate. B, P1 brain fraction shows a significant reduction in AT8 signal for PHF1 and a trend toward reduction for MC1 compared with control antibody. C, S1 brain fraction shows a significant reduction in AT8 signal for PHF1 and a trend toward reduction for MC1 compared with control antibody. Note that the AT8 signal in the soluble fraction is <10% of the P1 signal. D, P1 spinal cord fraction shows a significant reduction in AT8 signal for MC1 and a trend toward reduction for PHF1 compared with control antibody.
FIGURE 10.
Representative images taken from the spinal cord and brain stem (×20) from P301S mice treated with control, MC1, and PHF1 antibodies. The images show a reduction in PG5 staining in the MC1- and PHF1-treated mice compared with control antibody-treated mice.
FIGURE 11.
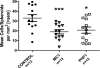
Quantitative analysis of pathology in the spinal cord and brain stem of P301S mice. A, quantitation of AT8-positive cells in brain stem shows a significant reduction for PHF1 and MC1 treatment compared with control antibody. There was also a significant reduction in of AT8 in the cord for PHF1-treated mice. B, quantitation of PG5 positive cells in brain stem and spinal cord shows a significant reduction for PHF1 and MC1 treatment compared with control antibody. C, quantitation of nY29-positive cells in spinal cord brain stem shows a trend toward reduction for PHF1 and MC1 treatment compared with control antibody. Data were analyzed using one-way ANOVA followed by post-hoc Dunnett test; *, p < 0.05 versus control antibody.
FIGURE 12.
Quantitative analysis of the number of neurofilament-positive axonal spheroids in the spinal cord of P301S mice. Data indicated that there was a significant reduction in NF200-positive neurospheroids in the spinal cord for PHF1 and MC1 treatment compared with control. Data were analyzed using one-way ANOVA followed by post-hoc Dunnett test. *, p < 0.05 versus control antibody.
Similar articles
- Passive immunization with phospho-tau antibodies reduces tau pathology and functional deficits in two distinct mouse tauopathy models.
Sankaranarayanan S, Barten DM, Vana L, Devidze N, Yang L, Cadelina G, Hoque N, DeCarr L, Keenan S, Lin A, Cao Y, Snyder B, Zhang B, Nitla M, Hirschfeld G, Barrezueta N, Polson C, Wes P, Rangan VS, Cacace A, Albright CF, Meredith J Jr, Trojanowski JQ, Lee VM, Brunden KR, Ahlijanian M. Sankaranarayanan S, et al. PLoS One. 2015 May 1;10(5):e0125614. doi: 10.1371/journal.pone.0125614. eCollection 2015. PLoS One. 2015. PMID: 25933020 Free PMC article. - Passive immunization targeting the N-terminal projection domain of tau decreases tau pathology and improves cognition in a transgenic mouse model of Alzheimer disease and tauopathies.
Dai CL, Chen X, Kazim SF, Liu F, Gong CX, Grundke-Iqbal I, Iqbal K. Dai CL, et al. J Neural Transm (Vienna). 2015 Apr;122(4):607-17. doi: 10.1007/s00702-014-1315-y. Epub 2014 Sep 19. J Neural Transm (Vienna). 2015. PMID: 25233799 - Vectored Intracerebral Immunization with the Anti-Tau Monoclonal Antibody PHF1 Markedly Reduces Tau Pathology in Mutant Tau Transgenic Mice.
Liu W, Zhao L, Blackman B, Parmar M, Wong MY, Woo T, Yu F, Chiuchiolo MJ, Sondhi D, Kaminsky SM, Crystal RG, Paul SM. Liu W, et al. J Neurosci. 2016 Dec 7;36(49):12425-12435. doi: 10.1523/JNEUROSCI.2016-16.2016. J Neurosci. 2016. PMID: 27927959 Free PMC article. - Immunotherapy targeting pathological tau protein in Alzheimer's disease and related tauopathies.
Sigurdsson EM. Sigurdsson EM. J Alzheimers Dis. 2008 Oct;15(2):157-68. doi: 10.3233/jad-2008-15202. J Alzheimers Dis. 2008. PMID: 18953105 Free PMC article. Review. - Anti-tau oligomers passive vaccination for the treatment of Alzheimer disease.
Kayed R. Kayed R. Hum Vaccin. 2010 Nov;6(11):931-5. doi: 10.4161/hv.6.11.12689. Epub 2010 Nov 1. Hum Vaccin. 2010. PMID: 20980799 Review.
Cited by
- GSK-3 and Tau: A Key Duet in Alzheimer's Disease.
Sayas CL, Ávila J. Sayas CL, et al. Cells. 2021 Mar 24;10(4):721. doi: 10.3390/cells10040721. Cells. 2021. PMID: 33804962 Free PMC article. Review. - Passive immunotherapy of tauopathy targeting pSer413-tau: a pilot study in mice.
Umeda T, Eguchi H, Kunori Y, Matsumoto Y, Taniguchi T, Mori H, Tomiyama T. Umeda T, et al. Ann Clin Transl Neurol. 2015 Mar;2(3):241-55. doi: 10.1002/acn3.171. Epub 2015 Jan 9. Ann Clin Transl Neurol. 2015. PMID: 25815351 Free PMC article. - Alzheimer disease therapeutics: focus on the disease and not just plaques and tangles.
Iqbal K, Liu F, Gong CX. Iqbal K, et al. Biochem Pharmacol. 2014 Apr 15;88(4):631-9. doi: 10.1016/j.bcp.2014.01.002. Epub 2014 Jan 10. Biochem Pharmacol. 2014. PMID: 24418409 Free PMC article. Review. - Specific serum antibody binding to phosphorylated and non-phosphorylated tau in non-cognitively impaired, mildly cognitively impaired, and Alzheimer's disease subjects: an exploratory study.
Klaver AC, Coffey MP, Bennett DA, Loeffler DA. Klaver AC, et al. Transl Neurodegener. 2017 Nov 24;6:32. doi: 10.1186/s40035-017-0100-x. eCollection 2017. Transl Neurodegener. 2017. PMID: 29204273 Free PMC article. - Innate immunity in Alzheimer's disease: the relevance of animal models?
Franco Bocanegra DK, Nicoll JAR, Boche D. Franco Bocanegra DK, et al. J Neural Transm (Vienna). 2018 May;125(5):827-846. doi: 10.1007/s00702-017-1729-4. Epub 2017 May 17. J Neural Transm (Vienna). 2018. PMID: 28516241 Free PMC article. Review.
References
- Thal D. R., Holzer M., Rüb U., Waldmann G., Günzel S., Zedlick D., Schober R. (2000) Exp. Neurol. 163, 98–110 - PubMed
- Hutton M., Lendon C. L., Rizzu P., Baker M., Froelich S., Houlden H., Pickering-Brown S., Chakraverty S., Isaacs A., Grover A., Hackett J., Adamson J., Lincoln S., Dickson D., Davies P., Petersen R. C., Stevens M., de Graaff E., Wauters E., van Baren J., Hillebrand M., Joosse M., Kwon J. M., Nowotny P., Che L. K., Norton J., Morris J. C., Reed L. A., Trojanowski J., Basun H., Lannfelt L., Neystat M., Fahn S., Dark F., Tannenberg T., Dodd P. R., Hayward N., Kwok J. B., Schofield P. R., Andreadis A., Snowden J., Craufurd D., Neary D., Owen F., Oostra B. A., Hardy J., Goate A., van Swieten J., Mann D., Lynch T., Heutink P. (1998) Nature 393, 702–705 - PubMed
- Goedert M., Klug A., Crowther R. (2006) J. Alzheimers Dis. 9, 195–207 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous