Enhancement of proteasomal function protects against cardiac proteinopathy and ischemia/reperfusion injury in mice - PubMed (original) (raw)
. 2011 Sep;121(9):3689-700.
doi: 10.1172/JCI45709. Epub 2011 Aug 15.
Affiliations
- PMID: 21841311
- PMCID: PMC3163952
- DOI: 10.1172/JCI45709
Enhancement of proteasomal function protects against cardiac proteinopathy and ischemia/reperfusion injury in mice
Jie Li et al. J Clin Invest. 2011 Sep.
Abstract
The ubiquitin-proteasome system degrades most intracellular proteins, including misfolded proteins. Proteasome functional insufficiency (PFI) has been observed in proteinopathies, such as desmin-related cardiomyopathy, and implicated in many common diseases, including dilated cardiomyopathy and ischemic heart disease. However, the pathogenic role of PFI has not been established. Here we created inducible Tg mice with cardiomyocyte-restricted overexpression of proteasome 28 subunit α (CR-PA28αOE) to investigate whether upregulation of the 11S proteasome enhances the proteolytic function of the proteasome in mice and, if so, whether the enhancement can rescue a bona fide proteinopathy and protect against ischemia/reperfusion (I/R) injury. We found that CR-PA28αOE did not alter the homeostasis of normal proteins and cardiac function, but did facilitate the degradation of a surrogate misfolded protein in the heart. By breeding mice with CR-PA28αOE with mice representing a well-established model of desmin-related cardiomyopathy, we demonstrated that CR-PA28αOE markedly reduced aberrant protein aggregation. Cardiac hypertrophy was decreased, and the lifespan of the animals was increased. Furthermore, PA28α knockdown promoted, whereas PA28α overexpression attenuated, accumulation of the mutant protein associated with desmin-related cardiomyopathy in cultured cardiomyocytes. Moreover, CR-PA28αOE limited infarct size and prevented postreperfusion cardiac dysfunction in mice with myocardial I/R injury. We therefore conclude that benign enhancement of cardiac proteasome proteolytic function can be achieved by CR-PA28αOE and that PFI plays a major pathogenic role in cardiac proteinopathy and myocardial I/R injury.
Figures
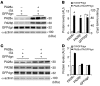
Figure 1. PA28α and PA28β expression in hearts of mice with CR-PA28αOE.
(A) 3 Tg responder lines carrying Tg PA28α were crossbred with the tTA Tg mice. No Dox was given to the breeding pairs or pups. Western blot analyses of 2-month-old mouse heart samples show that CR-PA28αOE resulted in a proportional increase of PA28β protein in the PA28α/tTA double-Tg mice. (B) Quantitative densitometry analyses of PA28α and PA28β protein levels. n = 3, *P < 0.001 vs. non-Tg (NTG); #P < 0.001 vs. tTA single-Tg. (C and D) Representative Northern blot analyses for PA28α (C) and PA28β (D). (E) Quantitative analysis of PA28β mRNA levels. CTL, control. n = 4. (F) RNA dot blot analyses of PA28α and PA28β transcript levels. GAPDH and ribosome 18S RNA served as loading controls. (G) Reciprocal IP with PA28α and PA28β antibodies showed increased PA28α-associated PA28β and PA28β-associated PA28α in myocardium with PA28αOE.
Figure 2. Effect of CR-PA28αOE on UPS proteolytic function in the heart.
Mice were generated via cross-breeding between those harboring homozygous GFPdgn and hemizygous tTA and those carrying hemizygous PA28α responder Tg. (A and B) Western blot analyses of PA28α and GFPdgn protein levels (n = 4 mice/group). *P < 0.001 vs. all other groups. (C–F) Semiquantitative RT-PCR (C) and RNA dot blot analyses (D and E) of steady-state GFPdgn transcript levels, and assessment of GFPdgn mRNA polysomal distribution (F), in the ventricles of PA28α/tTA/GFPdgn triple-Tg (PA28αOE) and tTA/GFPdgn double-Tg control littermates. (C and D) GAPDH was analyzed for the loading control. (F) Polysomes were isolated from ventricular myocardium using sucrose gradients (see Methods). RNAs were extracted from the gradient fractions and used for RT-PCR to detect the distribution of GFPdgn mRNA. PA28α and GAPDH were probed as positive and negative controls, respectively.
Figure 3. Inducible activation of UPS proteolytic function.
(A and B) Western blot analysis of PA28α, PA28β, and GFPdgn. Dox administration was started from the breeding pairs and withdrawn when the offspring reached 8 weeks of age. Cardiac tissue was collected 8 weeks after Dox withdrawal for the analyses. (C and D) For age-matched mice that received Dox treatment throughout, induced expression of PA28α was blocked, and GFPdgn protein levels were not significantly altered. Each lane was from an individual mouse. #P < 0.01, *P <0.05 vs. tTA/GFPdgn.
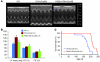
Figure 4. PA28αOE attenuates cardiac hypertrophy and delays premature death of mice with CryABR120G-based cardiomyopathy.
(A and B) Effect of CR-PA28αOE on LV mass, EF, and FS, assessed by echocardiography at 12 weeks (see Supplemental Table 3 for other parameters). mCryAB, CryABR120G. *P < 0.05 vs. tTA; #P < 0.05 vs. tTA/CryABR120G. (C) Survival rate of a cohort of mixed-sex littermate tTA/CryABR120G double-Tg or PA28α/tTA/CryABR120G triple-Tg mice was monitored daily, and survival data were used for Kaplan-Meier analysis. P < 0.01, log-rank test. In B and C, n is shown in parentheses.
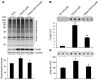
Figure 5. PA28αOE reduces CryABR120G-induced aberrant protein aggregation.
(A) Western blot analyses of total ubiquitinated proteins in mouse ventricular myocardium. Representative images and pooled densitometry data (n = 4 mice/group) are shown. Total CryAB protein levels were probed to verify CryABR120G overexpression in the PA28α/tTA/CryABR120G triple-Tg mice. α-actinin was probed as a loading control. *P < 0.05 vs. non-Tg; #P < 0.05 vs. tTA/CryABR120G. (B and C) Ventricular myocardium from WT CryAB Tg, tTA/CryABR120G double-Tg, or PA28α/tTA/CryABR120G triple-Tg mice was processed for the filter trapping assay (see Methods). The proteins retained on the filter were immunoprobed for CryAB (B) or ubiquitin (C). Summarized densitometry data are also shown. *P < 0.05, **P < 0.01 vs. WT CryAB; #P < 0.05, ##P < 0.01 vs. tTA/CryABR120G.
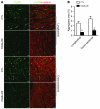
Figure 6. Confocal microscopic analysis of immunofluorescence-stained CryAB aggregates in DRC mouse hearts.
Cryosections of perfusion-fixed ventricular myocardium from PA28α/tTA/CryABR120G triple-Tg mice and tTA/CryABR120G double-Tg controls were used for immunofluorescence staining for CryAB (green) and α-actinin (red). (A) Representative images of longitudinal sections and cross-sections. Scale bars: 20 μm. (B) Morphometric quantification of CryAB-positive protein aggregates in myocardial sections from CryABR120G Tg hearts. *P < 0.05 vs. control.
Figure 7. Effects of genetic manipulation of PA28α on the stability of a bona fide misfolded protein in cultured NRCMs.
(A and B) PA28α knockdown was achieved via 2 consecutive transfections of siRNA against rat PA28α; siRNA for luciferase (siRNA-Luc) was used as control. (C and D) PA28α or HA-tagged CryABR120G overexpression was achieved by infection of Ad-PA28α and Ad–HA-CryABR120G (Ad-HA-R120G), respectively; Ad–β-gal was used as control. Manipulations of PA28α were performed 24 hours before initiation of HA-CryABR120G overexpression. Cells were collected for protein and RNA extractions 4 days after Ad–HA-CryABR120G infection. (A and C) Representative Western blot (IB) images of the indicated proteins. A longer exposure (long expo.) of IB for HA-tag illustrates that a higher–molecular weight species of HA-CryABR120G in the insoluble fraction was also altered by changing PA28α expression. (B and D) Changes in HA-CryABR120G protein levels by PA28α knockdown (B) or PA28αOE (D). Shown are HA-CryABR120G protein levels normalized with the corresponding in-lane loading control, GAPDH or α-actinin. *P < 0.05. (E) Representative RT-PCR images for HA-CryABR120G in NRCMs. PA28αOE did not alter HA-CryABR120G mRNA levels compared with controls.
Figure 8. Enhancing cardiac proteasome function protects against myocardial I/R injury.
I/R injuries were created on tTA single-Tg control or PA28α/tTA double-Tg mice by left anterior descending artery ligation (30 minutes) and release. (A–D) A pressure transducer catheter was inserted into LV via the carotid artery, and LV pressure and dP/dt were monitored. Shown are (A) LVSP, (B) dP/dtmax, (C) dP/dtmin, and (D) HR at baseline, 30 minutes after left anterior descending artery ligation (Isc), and 30 and 45 minutes after reperfusion (Rep). n = 6 or 7 mice/group. *P < 0.05. (E and F) Myocardial ischemia and infarct size were assessed at 24 hours of reperfusion. Phthalocyanine blue perfusion after left anterior descending artery religation at the terminal experiment defined the AAR as the area not perfused. Within the AAR, triphenyltetrazolium chloride staining demarcated the infarcted area (IA; white) and viable (red) myocardium. Shown are representative section series images (E) and quantitative data (F). Scale bar: 10 mm. **P < 0.01.
Similar articles
- Inadequate ubiquitination-proteasome coupling contributes to myocardial ischemia-reperfusion injury.
Hu C, Tian Y, Xu H, Pan B, Terpstra EM, Wu P, Wang H, Li F, Liu J, Wang X. Hu C, et al. J Clin Invest. 2018 Dec 3;128(12):5294-5306. doi: 10.1172/JCI98287. Epub 2018 Oct 22. J Clin Invest. 2018. PMID: 30204128 Free PMC article. - Genetically induced moderate inhibition of the proteasome in cardiomyocytes exacerbates myocardial ischemia-reperfusion injury in mice.
Tian Z, Zheng H, Li J, Li Y, Su H, Wang X. Tian Z, et al. Circ Res. 2012 Aug 17;111(5):532-42. doi: 10.1161/CIRCRESAHA.112.270983. Epub 2012 Jun 26. Circ Res. 2012. PMID: 22740087 Free PMC article. - Enhancement of proteasome function by PA28α overexpression protects against oxidative stress.
Li J, Powell SR, Wang X. Li J, et al. FASEB J. 2011 Mar;25(3):883-93. doi: 10.1096/fj.10-160895. Epub 2010 Nov 23. FASEB J. 2011. PMID: 21098724 Free PMC article. - The ubiquitin-proteasome system in cardiac proteinopathy: a quality control perspective.
Su H, Wang X. Su H, et al. Cardiovasc Res. 2010 Jan 15;85(2):253-62. doi: 10.1093/cvr/cvp287. Epub 2009 Aug 20. Cardiovasc Res. 2010. PMID: 19696071 Free PMC article. Review. - Proteasome functional insufficiency in cardiac pathogenesis.
Wang X, Li J, Zheng H, Su H, Powell SR. Wang X, et al. Am J Physiol Heart Circ Physiol. 2011 Dec;301(6):H2207-19. doi: 10.1152/ajpheart.00714.2011. Epub 2011 Sep 23. Am J Physiol Heart Circ Physiol. 2011. PMID: 21949118 Free PMC article. Review.
Cited by
- Proteostasis and Its Role in Disease Development.
Shukla M, Narayan M. Shukla M, et al. Cell Biochem Biophys. 2024 Oct 18. doi: 10.1007/s12013-024-01581-6. Online ahead of print. Cell Biochem Biophys. 2024. PMID: 39422790 - Genetic blockade of the activation of 26S proteasomes by PKA is well tolerated by mice at baseline.
Yang L, Ahammed MS, Wu P, Sternburg JO, Liu J, Wang X. Yang L, et al. Am J Cardiovasc Dis. 2024 Apr 15;14(2):90-105. doi: 10.62347/NSWR6869. eCollection 2024. Am J Cardiovasc Dis. 2024. PMID: 38764549 Free PMC article. - Cardiac proteostasis in obesity and cardiovascular disease.
Guerra J, Matta L, Bartelt A. Guerra J, et al. Herz. 2024 Mar;49(2):118-123. doi: 10.1007/s00059-024-05233-6. Epub 2024 Feb 8. Herz. 2024. PMID: 38329532 Free PMC article. Review. - S14-Phosphorylated RPN6 Mediates Proteasome Activation by PKA and Alleviates Proteinopathy.
Yang L, Parajuli N, Wu P, Liu J, Wang X. Yang L, et al. Circ Res. 2023 Sep 15;133(7):572-587. doi: 10.1161/CIRCRESAHA.123.322887. Epub 2023 Aug 29. Circ Res. 2023. PMID: 37641975 Free PMC article.
References
- Scruggs SB, Ping P, Zong C. Heterogeneous cardiac proteasomes: mandated by diverse substrates? Physiology (Bethesda). 2011;26(2):106–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL068936/HL/NHLBI NIH HHS/United States
- R01HL072166/HL/NHLBI NIH HHS/United States
- R01 HL085629/HL/NHLBI NIH HHS/United States
- R01HL085629/HL/NHLBI NIH HHS/United States
- R01HL068936/HL/NHLBI NIH HHS/United States
- R01 HL072166/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous