Triheptanoin--a medium chain triglyceride with odd chain fatty acids: a new anaplerotic anticonvulsant treatment? - PubMed (original) (raw)
Review
Triheptanoin--a medium chain triglyceride with odd chain fatty acids: a new anaplerotic anticonvulsant treatment?
Karin Borges et al. Epilepsy Res. 2012 Jul.
Abstract
The triglyceride of heptanoate (C7 fatty acid), triheptanoin, is a tasteless oil used to treat rare metabolic disorders in USA and France. Heptanoate is metabolized by β-oxidation to provide propionyl-CoA, which after carboxylation can produce succinyl-CoA, resulting in anaplerosis - the refilling of the tricarboxylic acid cycle. Heptanoate is also metabolized by the liver to the C5 ketones, β-ketopentanoate and/or β-hydroxypentanoate, which are released into the blood and thought to enter the brain via monocarboxylate transporters. Oral triheptanoin has recently been discovered to be reproducibly anticonvulsant in acute and chronic mouse seizures models. However, current knowledge on alterations of brain metabolism after triheptanoin administration and anaplerosis via propionyl-CoA carboxylation in the brain is limited. This review outlines triheptanoin's unique anticonvulsant profile and its clinical potential for the treatment of medically refractory epilepsy. Anaplerosis as a therapeutic approach for the treatment of epilepsy is discussed. More research is needed to elucidate the anticonvulsant mechanism of triheptanoin and to reveal its clinical potential for the treatment of epilepsy and other disorders of the brain.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures
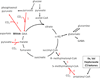
Fig. 1
Anaplerotic pathways. Enzymes are marked by numbers. In the brain, the main anaplerotic pathway is dependent on pyruvate carboxylase (1). The “reverse” reaction from malate is catalysed by malic enzyme, 2). Phosphoenol-pyruvate carboxykinase (3) is considered to work in the decarboyxlation direction. Anaplerosis via the propionyl-CoA carboxylation pathway using branched chain amino acids (BCAA), heptanoate or the “C5 ketones“β-ketopentanoate and β-hydroxypentanoate involves propionyl-CoA carboxylase (4), methylmalonyl-CoA epimerase (5) and methylmalonyl-CoA mutase (6). Abbreviations: BCAA – branched chain amino acids, α-KG – α-ketoglutarate, OAA- oxaloacetate, Co-A-coenzyme A
Similar articles
- Anticonvulsant effects of a triheptanoin diet in two mouse chronic seizure models.
Willis S, Stoll J, Sweetman L, Borges K. Willis S, et al. Neurobiol Dis. 2010 Dec;40(3):565-72. doi: 10.1016/j.nbd.2010.07.017. Epub 2010 Aug 4. Neurobiol Dis. 2010. PMID: 20691264 Free PMC article. - Triheptanoin in acute mouse seizure models.
Thomas NK, Willis S, Sweetman L, Borges K. Thomas NK, et al. Epilepsy Res. 2012 May;99(3):312-7. doi: 10.1016/j.eplepsyres.2011.12.013. Epub 2012 Jan 20. Epilepsy Res. 2012. PMID: 22260920 - Modification of Astrocyte Metabolism as an Approach to the Treatment of Epilepsy: Triheptanoin and Acetyl-L-Carnitine.
Hadera MG, McDonald T, Smeland OB, Meisingset TW, Eloqayli H, Jaradat S, Borges K, Sonnewald U. Hadera MG, et al. Neurochem Res. 2016 Feb;41(1-2):86-95. doi: 10.1007/s11064-015-1728-5. Epub 2015 Oct 3. Neurochem Res. 2016. PMID: 26433381 Review. - Triheptanoin partially restores levels of tricarboxylic acid cycle intermediates in the mouse pilocarpine model of epilepsy.
Hadera MG, Smeland OB, McDonald TS, Tan KN, Sonnewald U, Borges K. Hadera MG, et al. J Neurochem. 2014 Apr;129(1):107-19. doi: 10.1111/jnc.12610. Epub 2013 Dec 2. J Neurochem. 2014. PMID: 24236946 - Alternative Fuels in Epilepsy and Amyotrophic Lateral Sclerosis.
Tefera TW, Tan KN, McDonald TS, Borges K. Tefera TW, et al. Neurochem Res. 2017 Jun;42(6):1610-1620. doi: 10.1007/s11064-016-2106-7. Epub 2016 Nov 21. Neurochem Res. 2017. PMID: 27868154 Review.
Cited by
- Oleaginous Microbial Lipids' Potential in the Prevention and Treatment of Neurological Disorders.
Alhattab M, Moorthy LS, Patel D, Franco CMM, Puri M. Alhattab M, et al. Mar Drugs. 2024 Feb 6;22(2):80. doi: 10.3390/md22020080. Mar Drugs. 2024. PMID: 38393051 Free PMC article. Review. - Microbial synthesis of functional odd-chain fatty acids: a review.
Zhang LS, Liang S, Zong MH, Yang JG, Lou WY. Zhang LS, et al. World J Microbiol Biotechnol. 2020 Feb 22;36(3):35. doi: 10.1007/s11274-020-02814-5. World J Microbiol Biotechnol. 2020. PMID: 32088779 Review. - Open-label long-term treatment of add-on triheptanoin in adults with drug-resistant epilepsy.
Borges K, Kaul N, Germaine J, Carrasco-Pozo C, Kwan P, O'Brien TJ. Borges K, et al. Epilepsia Open. 2020 Apr 12;5(2):230-239. doi: 10.1002/epi4.12391. eCollection 2020 Jun. Epilepsia Open. 2020. PMID: 32524048 Free PMC article. - Enrichment of C17:0-rich saturated fatty acids from sheep tail fat for adjuvant therapy of non-small-cell lung cancer.
Yu X, Liu X, Li Y, He H, Pei X, Ma T, Chen Y, Wang Y, Li H, Lin W, Xu C, Zhang B. Yu X, et al. Food Sci Biotechnol. 2024 Jan 23;33(8):1947-1956. doi: 10.1007/s10068-023-01504-w. eCollection 2024 Jun. Food Sci Biotechnol. 2024. PMID: 38752121 - Ketone Administration for Seizure Disorders: History and Rationale for Ketone Esters and Metabolic Alternatives.
Poff AM, Rho JM, D'Agostino DP. Poff AM, et al. Front Neurosci. 2019 Oct 15;13:1041. doi: 10.3389/fnins.2019.01041. eCollection 2019. Front Neurosci. 2019. PMID: 31680801 Free PMC article. Review.
References
- Alvestad S, Hammer J, Eyjolfsson E, Qu H, Ottersen OP, Sonnewald U. Limbic structures show altered glial-neuronal metabolism in the chronic phase of kainate induced epilepsy. Neurochem. Res. 2008;33:257–266. - PubMed
- Bak LK, Iversen P, Sorensen M, Keiding S, Vilstrup H, Ott P, Waagepetersen HS, Schousboe A. Metabolic fate of isoleucine in a rat model of hepatic encephalopathy and in cultured neural cells exposed to ammonia. Metab. Brain Dis. 2009;24:135–145. - PubMed
- Ballhausen D, Mittaz L, Boulat O, Bonafe L, Braissant O. Evidence for catabolic pathway of propionate metabolism in CNS: expression pattern of methylmalonyl-CoA mutase and propionyl-CoA carboxylase alpha-subunit in developing and adult rat brain. Neuroscience. 2009;164:578–587. - PubMed
- Barton ME, Klein BD, Wolf HH, White HS. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001;47:217–227. - PubMed
- Blanco MM, dos Santos JG, Jr, Perez-Mendes P, Kohek SR, Cavarsan CF, Hummel M, Albuquerque C, Mello LE. Assessment of seizure susceptibility in pilocarpine epileptic and nonepileptic Wistar rats and of seizure reinduction with pentylenetetrazole and electroshock models. Epilepsia. 2009;50:824–831. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous