Molecular bases of corneal endothelial dystrophies - PubMed (original) (raw)
Review
Molecular bases of corneal endothelial dystrophies
Thore Schmedt et al. Exp Eye Res. 2012 Feb.
Abstract
The phrase "corneal endothelial dystrophies" embraces a group of bilateral corneal conditions that are characterized by a non-inflammatory and progressive degradation of corneal endothelium. Corneal endothelial cells exhibit a high pump site density and, along with barrier function, are responsible for maintaining the cornea in its natural state of relative dehydration. Gradual loss of endothelial cells leads to an insufficient water outflow, resulting in corneal edema and loss of vision. Since the pathologic mechanisms remain largely unknown, the only current treatment option is surgical transplantation when vision is severely impaired. In the past decade, important steps have been taken to understand how endothelial degeneration progresses on the molecular level. Studies of affected multigenerational families and sporadic cases identified genes and chromosomal loci, and revealed either Mendelian or complex disorder inheritance patterns. Mutations have been detected in genes that carry important structural, metabolic, cytoprotective, and regulatory functions in corneal endothelium. In addition to genetic predisposition, environmental factors like oxidative stress were found to be involved in the pathogenesis of endotheliopathies. This review summarizes and crosslinks the recent progress on deciphering the molecular bases of corneal endothelial dystrophies.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
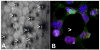
Fig. 1
A. Confocal microscopy of a patient with FECD. Black areas (arrowheads) represent corneal guttae that are scattered in between corneal endothelial cells, disrupting a normally continuous layer of hexagonally shaped cells. B. Magnified view of one corneal gutta (arrowhead) with a center devoid of corneal endothelial cells. The remaining endothelial cells cluster around the gutta, staining positive for TUNEL (red), a marker of apoptosis, and positive for 8-OHdG (green), a marker of oxidative DNA damage.
Fig. 2
Topology model for human SLC4All. Numbers indicate amino acid position. Predicted N-glycosylation sites are in black, and the branched structures represent oligosaccharide moieties. Black and gray arrowheads indicate trypsin cleavage sites identified through partial digestion of Myc-SLC4A11 and SLC4A11-Myc, respectively, as described in Vilas et al., 2011 (Vilas, G.L. et al., 2011). Identified point mutations causing CHED2 (blue filled), FECD (red filled), and Harboyan syndrome (orange filled) are indicated (see also Vilas et al. 2011, Refs. 4-6, 11,12,34,37,39,41,42). S213 was identified as mutated in both Harboyan syndrome and CHED2 and is shown in filled blue and orange, accordingly. Asterisks indicate residues where two different point mutations have been found to cause disease.
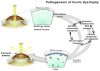
Fig. 3
Diagram of the pathogenesis of FECD. Oxidative stress and genetic factors combined with endothelial cell post-mitotic arrest may lead to oxidant-antioxidant imbalance, oxidative mitochondrial DNA damage, endothelial morphological changes and apoptosis, and cause the corneal edema seen in FECD.
Fig. 4
Specular photomicrographs of normal (A) and PPCD endothelium (B-E). PPCD endothelium displays pleomorphism, polymegethism, and vesicular lesions. Light microscopy detects oen layer of regular flat cells in normal endothelium (F). In PPCD, endothelium is composed of multilayered cells with prominent round nuclei and numerous projections (G,H). Red-propidium iodide. Courtesy of P. Liskova, M.D., Laboratory of the Biology and Pathology of the Eye, Charles University, Prague).
Similar articles
- Missense mutations in COL8A2, the gene encoding the alpha2 chain of type VIII collagen, cause two forms of corneal endothelial dystrophy.
Biswas S, Munier FL, Yardley J, Hart-Holden N, Perveen R, Cousin P, Sutphin JE, Noble B, Batterbury M, Kielty C, Hackett A, Bonshek R, Ridgway A, McLeod D, Sheffield VC, Stone EM, Schorderet DF, Black GC. Biswas S, et al. Hum Mol Genet. 2001 Oct 1;10(21):2415-23. doi: 10.1093/hmg/10.21.2415. Hum Mol Genet. 2001. PMID: 11689488 - Transcriptome analysis of the human corneal endothelium.
Frausto RF, Wang C, Aldave AJ. Frausto RF, et al. Invest Ophthalmol Vis Sci. 2014 Nov 6;55(12):7821-30. doi: 10.1167/iovs.14-15021. Invest Ophthalmol Vis Sci. 2014. PMID: 25377225 Free PMC article. - [Mutational analysis of VSX-1 in one patient with posterior polymorphous corneal dystrophy and in three families with hereditary Fuchs endothelial dystrophy].
Clausen I, Weidle E, Duncker G, Grünauer-Kloevekorn C. Clausen I, et al. Klin Monbl Augenheilkd. 2009 Jun;226(6):466-9. doi: 10.1055/s-0028-1109427. Epub 2009 Jun 8. Klin Monbl Augenheilkd. 2009. PMID: 19507099 German. - Update on the genetics of corneal endothelial dystrophies.
Kannabiran C, Chaurasia S, Ramappa M, Mootha VV. Kannabiran C, et al. Indian J Ophthalmol. 2022 Jul;70(7):2239-2248. doi: 10.4103/ijo.IJO_992_22. Indian J Ophthalmol. 2022. PMID: 35791103 Free PMC article. Review. - Fuchs' dystrophy.
Wilson SE, Bourne WM. Wilson SE, et al. Cornea. 1988;7(1):2-18. Cornea. 1988. PMID: 3280235 Review.
Cited by
- Rescue of the Congenital Hereditary Endothelial Dystrophy Mouse Model by Adeno-Associated Viruse-Mediated Slc4a11 Replacement.
Shyam R, Ogando DG, Kim ET, Murugan S, Choi M, Bonanno JA. Shyam R, et al. Ophthalmol Sci. 2022 Mar;2(1):100084. doi: 10.1016/j.xops.2021.100084. Epub 2021 Nov 23. Ophthalmol Sci. 2022. PMID: 36051248 Free PMC article. - Novel Identity and Functional Markers for Human Corneal Endothelial Cells.
Bartakova A, Alvarez-Delfin K, Weisman AD, Salero E, Raffa GA, Merkhofer RM Jr, Kunzevitzky NJ, Goldberg JL. Bartakova A, et al. Invest Ophthalmol Vis Sci. 2016 May 1;57(6):2749-62. doi: 10.1167/iovs.15-18826. Invest Ophthalmol Vis Sci. 2016. PMID: 27196322 Free PMC article. - Endothelial cell whole genome expression analysis in a mouse model of early-onset Fuchs' endothelial corneal dystrophy.
Matthaei M, Hu J, Meng H, Lackner EM, Eberhart CG, Qian J, Hao H, Jun AS. Matthaei M, et al. Invest Ophthalmol Vis Sci. 2013 Mar 15;54(3):1931-40. doi: 10.1167/iovs.12-10898. Invest Ophthalmol Vis Sci. 2013. PMID: 23449721 Free PMC article. - Wound-Induced Polyploidization: Regulation by Hippo and JNK Signaling and Conservation in Mammals.
Losick VP, Jun AS, Spradling AC. Losick VP, et al. PLoS One. 2016 Mar 9;11(3):e0151251. doi: 10.1371/journal.pone.0151251. eCollection 2016. PLoS One. 2016. PMID: 26958853 Free PMC article. - Magnetic field-guided cell delivery with nanoparticle-loaded human corneal endothelial cells.
Moysidis SN, Alvarez-Delfin K, Peschansky VJ, Salero E, Weisman AD, Bartakova A, Raffa GA, Merkhofer RM Jr, Kador KE, Kunzevitzky NJ, Goldberg JL. Moysidis SN, et al. Nanomedicine. 2015 Apr;11(3):499-509. doi: 10.1016/j.nano.2014.12.002. Epub 2015 Jan 14. Nanomedicine. 2015. PMID: 25596075 Free PMC article.
References
- Adamis AP, Filatov V, Tripathi BJ, Tripathi RC. Fuchs’ endothelial dystrophy of the cornea. Surv Ophthalmol. 1993;38:149–168. - PubMed
- Aldave AJ, Sonmez B. Elucidating the molecular genetic basis of the corneal dystrophies: are we there yet? Arch Ophthalmol. 2007;125:177–186. - PubMed
- Aldave AJ, Yellore VS, Bourla N, Momi RS, Khan MA, Salem AK, Rayner SA, Glasgow BJ, Kurtz I. Autosomal recessive CHED associated with novel compound heterozygous mutations in SLC4A11. Cornea. 2007a;26:896–900. - PubMed
- Aldave AJ, Yellore VS, Principe AH, Abedi G, Merrill K, Chalukya M, Small KW, Udar N. Candidate gene screening for posterior polymorphous dystrophy. Cornea. 2005;24:151–155. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous