Post-translational modifications of Hsp90 and their contributions to chaperone regulation - PubMed (original) (raw)
Review
Post-translational modifications of Hsp90 and their contributions to chaperone regulation
Mehdi Mollapour et al. Biochim Biophys Acta. 2012 Mar.
Abstract
Molecular chaperones, as the name suggests, are involved in folding, maintenance, intracellular transport, and degradation of proteins as well as in facilitating cell signaling. Heat shock protein 90 (Hsp90) is an essential eukaryotic molecular chaperone that carries out these processes in normal and cancer cells. Hsp90 function in vivo is coupled to its ability to hydrolyze ATP and this can be regulated by co-chaperones and post-translational modifications. In this review, we explore the varied roles of known post-translational modifications of cytosolic and nuclear Hsp90 (phosphorylation, acetylation, S-nitrosylation, oxidation and ubiquitination) in fine-tuning chaperone function in eukaryotes. This article is part of a Special Issue entitled: Heat Shock Protein 90 (HSP90).
Published by Elsevier B.V.
Figures
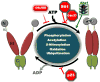
Figure 1
Post-translational modification of Hsp90 fine-tunes its chaperone function. ATP binding to the N-terminal domain of Hsp90 (gray) promotes transient dimerization of the N-domains (closed conformation). Subsequent structural rearrangements establish the ’closed and twisted’ conformation capable of ATP hydrolysis. The co-chaperone Aha1 enhances Hsp90 ATPase activity by facilitating the conformational changes necessary to achieve ATPase competence, while Sti1 and Hsp90 inhibitors such as geldanamycin (GA) or radicicol (RD) exert the opposite effect by inhibiting the initial structural changes necessary for N-domain dimerization. p23 slows ATP hydrolysis at a late stage of the chaperone cycle. Domain labeling is as follows: N, N-domain (gray); CL, charged linker (yellow); M, M-domain (amber); C, C-domain (green).
Figure 2
Post-translational modification sites on Hsp90. Domain location of phosphorylated serine (S), theronine (T) and tyrosine (Y) sites for which kinases are known, acetylated lysine (K) residues (pale blue), S-nitrosylated cysteine (C) (green) and cysteine oxidation sites (brown) on human Hsp90α and Hsp90β are shown. For additional phosphorylation sites, the reader is referred to [64,76].
Similar articles
- Post-translational modifications of Hsp90 and translating the chaperone code.
Backe SJ, Sager RA, Woodford MR, Makedon AM, Mollapour M. Backe SJ, et al. J Biol Chem. 2020 Aug 7;295(32):11099-11117. doi: 10.1074/jbc.REV120.011833. Epub 2020 Jun 11. J Biol Chem. 2020. PMID: 32527727 Free PMC article. Review. - The Hsp90 chaperone machinery: conformational dynamics and regulation by co-chaperones.
Li J, Soroka J, Buchner J. Li J, et al. Biochim Biophys Acta. 2012 Mar;1823(3):624-35. doi: 10.1016/j.bbamcr.2011.09.003. Epub 2011 Sep 16. Biochim Biophys Acta. 2012. PMID: 21951723 Review. - Detecting Posttranslational Modifications of Hsp90 Isoforms.
Sager RA, Backe SJ, Neckers L, Woodford MR, Mollapour M. Sager RA, et al. Methods Mol Biol. 2023;2693:125-139. doi: 10.1007/978-1-0716-3342-7_11. Methods Mol Biol. 2023. PMID: 37540432 Free PMC article. - The 'active life' of Hsp90 complexes.
Prodromou C. Prodromou C. Biochim Biophys Acta. 2012 Mar;1823(3):614-23. doi: 10.1016/j.bbamcr.2011.07.020. Epub 2011 Aug 4. Biochim Biophys Acta. 2012. PMID: 21840346 Free PMC article. Review. - Contributions of co-chaperones and post-translational modifications towards Hsp90 drug sensitivity.
Walton-Diaz A, Khan S, Bourboulia D, Trepel JB, Neckers L, Mollapour M. Walton-Diaz A, et al. Future Med Chem. 2013 Jun;5(9):1059-71. doi: 10.4155/fmc.13.88. Future Med Chem. 2013. PMID: 23734688 Free PMC article. Review.
Cited by
- Host cell stress response as a predictor of COVID-19 infectivity and disease progression.
Caillet C, Stofberg ML, Muleya V, Shonhai A, Zininga T. Caillet C, et al. Front Mol Biosci. 2022 Aug 11;9:938099. doi: 10.3389/fmolb.2022.938099. eCollection 2022. Front Mol Biosci. 2022. PMID: 36032680 Free PMC article. Review. - In Vivo Conformational Dynamics of Hsp90 and Its Interactors.
Chavez JD, Schweppe DK, Eng JK, Bruce JE. Chavez JD, et al. Cell Chem Biol. 2016 Jun 23;23(6):716-26. doi: 10.1016/j.chembiol.2016.05.012. Cell Chem Biol. 2016. PMID: 27341434 Free PMC article. - Dynamic tyrosine phosphorylation modulates cycling of the HSP90-P50(CDC37)-AHA1 chaperone machine.
Xu W, Mollapour M, Prodromou C, Wang S, Scroggins BT, Palchick Z, Beebe K, Siderius M, Lee MJ, Couvillon A, Trepel JB, Miyata Y, Matts R, Neckers L. Xu W, et al. Mol Cell. 2012 Aug 10;47(3):434-43. doi: 10.1016/j.molcel.2012.05.015. Epub 2012 Jun 21. Mol Cell. 2012. PMID: 22727666 Free PMC article. - PINCH in the cellular stress response to tau-hyperphosphorylation.
Ozdemir AY, Rom I, Kovalevich J, Yen W, Adiga R, Dave RS, Langford D. Ozdemir AY, et al. PLoS One. 2013;8(3):e58232. doi: 10.1371/journal.pone.0058232. Epub 2013 Mar 12. PLoS One. 2013. PMID: 23554879 Free PMC article. Clinical Trial. - Histone deacetylase inhibitor (HDACi) suberoylanilide hydroxamic acid (SAHA)-mediated correction of α1-antitrypsin deficiency.
Bouchecareilh M, Hutt DM, Szajner P, Flotte TR, Balch WE. Bouchecareilh M, et al. J Biol Chem. 2012 Nov 2;287(45):38265-78. doi: 10.1074/jbc.M112.404707. Epub 2012 Sep 20. J Biol Chem. 2012. PMID: 22995909 Free PMC article.
References
- Martin J. Chaperonin function--effects of crowding and confinement. J Mol Recognit. 2004;17:465–472. - PubMed
- Kampinga HH. Chaperones in preventing protein denaturation in living cells and protecting against cellular stress. Handb Exp Pharmacol. 2006:1–42. - PubMed
- Wandinger SK, Richter K, Buchner J. The Hsp90 chaperone machinery. J Biol Chem. 2008;283:18473–18477. - PubMed
- Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- ZIA SC010074-14/ImNIH/Intramural NIH HHS/United States
- ZIA SC010074-15/ImNIH/Intramural NIH HHS/United States
- ZIA BC011032-03/ImNIH/Intramural NIH HHS/United States
- Z01 SC010074-12/ImNIH/Intramural NIH HHS/United States
- Z99 CA999999/ImNIH/Intramural NIH HHS/United States
- Z01 BC011032/ImNIH/Intramural NIH HHS/United States
- Z01 BC011032-01/ImNIH/Intramural NIH HHS/United States
- Y99 CA999999/CA/NCI NIH HHS/United States
- Z01 SC010074/ImNIH/Intramural NIH HHS/United States
- ZIA BC011032-02/ImNIH/Intramural NIH HHS/United States
- Z01 SC010074-13/ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources