Using simple donors to drive the equilibria of glycosyltransferase-catalyzed reactions - PubMed (original) (raw)
Using simple donors to drive the equilibria of glycosyltransferase-catalyzed reactions
Richard W Gantt et al. Nat Chem Biol. 2011.
Abstract
We report that simple glycoside donors can drastically shift the equilibria of glycosyltransferase-catalyzed reactions, transforming NDP-sugar formation from an endothermic to an exothermic process. To demonstrate the utility of this thermodynamic adaptability, we highlight the glycosyltransferase-catalyzed synthesis of 22 sugar nucleotides from simple aromatic sugar donors, as well as the corresponding in situ formation of sugar nucleotides as a driving force in the context of glycosyltransferase-catalyzed reactions for small-molecule glycodiversification. These simple aromatic donors also enabled a general colorimetric assay for glycosyltransfer, applicable to drug discovery, protein engineering and other fundamental sugar nucleotide-dependent investigations. This study directly challenges the general notion that NDP-sugars are 'high-energy' sugar donors when taken out of their traditional biological context.
Conflict of interest statement
Competing financial interests
The authors report competing interests. J.S.T. is a co-founder of Centrose (Madison, WI, USA).
Figures
Fig. 1
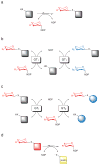
Representative GT-catalyzed reactions. a) Classical GT-catalyzed transformation wherein the sugar, presented in the form of a sugar nucleotide donor, is conjugated to an acceptor target of interest to provide a thermodynamically-favored glycoside product. b) A GT-catalyzed ‘sugar exchange’ reaction. In this reaction, a small amount of NDP is used to ‘prime’ the removal of the endogenous sugar appendage of a target complex natural product thus enabling the exchange of a native sugar for an endogenous sugar supplied in vast excess as a sugar nucleotide. c) A GT-catalyzed ‘aglycon exchange’ reaction where the sugar from one complex natural product is excised (using excess NDP) and subsequently attached to a structurally distinct target aglycon. d) The present study demonstrates the use of simple activated glycosides to dramatically shift the thermodynamics of GT-catalyzed reactions and thereby drive GT-catalyzed NDP-sugar synthesis, sugar exchange/and or aglycon exchange reactions while also offering a convenient colorimetric screen for glycosylation.
Fig. 2
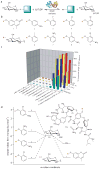
Evaluation of putative donors for sugar nucleotide synthesis. (a) General reaction scheme. (b) Structures of the β-D-glucopyranoside donors which led to (U/T)DP-glucose formation. (c) Percent conversion of (U/T)DP to (U/T)DP-glucose with various donors (n ≥ 2, standard deviation ≤ 5%). Reactions contained 2.1 μM (10 μg) OleD variant, 1 mM of (U/T)DP, and 1 mM of aromatic donor (1–9) in Tris-HCl buffer (50 mM, pH 8.5) with a final volume of 100 μl. After one hour at 25°C, reactions were flash frozen and analyzed by HPLC (Supplementary Methods). The pKa for each corresponding donor aglycon is highlighted in parentheses. (d) Plot depicting the relative Gibbs free energy of selected donors/acceptors in relation to 33a. Small glycoside donors display large shifts in relative free energy, transforming formation of UDP-Glc (33a) from an endo- to an exothermic process. The ΔG°pH8.5 for 1, 2, 4, 7, and 9 with UDP in Tris-HCl buffer (50 mM, pH 8.5) at 298K relative to 33a were determined in this study (Supplementary Methods). The ΔG° for 61a was previously determined (at pH 9.0 and 310K)(5).
Fig. 3
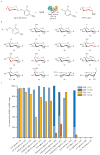
The synthesis of sugar nucleotides from 2-chloro-4-nitrophenyl glucosides. (a) General reaction scheme. (b) Structures of 2-chloro-4-nitrophenyl glycoside donors evaluated for D-sugars within this series, the differences between each member and the native OleD sugar substrate (β-D-glucose) are highlighted in red. (c) Maximum observed percent conversion of (U/T)DP to (U/T)DP-glucose within a 21 hour time course assay for each donor (n ≥ 2, standard deviation ≤ 5%). Standard reactions contained 7 μM TDP-16, 1 mM (U/T)DP, and 1 mM of 2-chloro-4-nitrophenyl glycoside donor (9, 34–47) in Tris-HCl buffer (50 mM, pH 8.5) with a final volume of 300 μl. Over 21 hours at 25°C, aliquots taken at various times were flash frozen and analyzed by HPLC (Supplementary Methods). For reactions with UDP yielding <45% conversion under standard conditions (40, 41, 43–47), identical assays using 10-fold less (U/T)DP (0.1 mM) were also conducted and, where relevant, the percent conversions for the modified reactions are represented by the darker colors. HPLC chromatograms, full time course data, and product characterization are presented in Supplementary Fig. 9–12 and Supplementary Table 3. In all cases where both the α- and β-anomers were examined as donors, only the β-anomer was found to be a substrate (Supplementary Methods and Supplementary Results).
Fig. 4
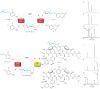
Evaluation of 2-chloro-4-nitrophenyl glycosides as sugar donors in coupled GT-catalyzed transglycosylation reactions. (a) The scheme for a single enzyme (TDP-16) coupled system with 4-methylumbelliferone (58) as the final acceptor (left) and a representative HPLC analysis (right) using the donor for 6-azido-6-deoxy-D-glucose (37). Reactions contained 1 mM glycoside donor, 1 mM 58, 1 mM UDP, and 11 μM TDP-16 in a total volume of 100 μl with Tris-HCl buffer (50 mM, pH 8.5) at 25°C for 24 hour and were subsequently analyzed by HPLC (Supplementary Methods). For the representative reaction: (i) control reaction lacking TDP-16; (ii) control reaction lacking UDP; (iii) full reaction where 37 is donor, 58 is acceptor, 59d is desired product and ⋄ represents 2-chloro-4-nitrophenolate. (b) The scheme for a double enzyme (TDP-16 and GtfE) coupled system with vancomycin aglycon (60) as the final acceptor (left) and a representative HPLC analysis (right) using the donor for 6-azido-6-deoxy-D-glucose (37). Reactions contained 1 mM glycoside donor, 0.1 mM 60, 1 mM UDP, 11 μM TDP-16, and 11 μM GtfE in a total volume of 100 μl with Tris-HCl buffer (50 mM, pH 8.5) at 25°C for 24 hour and were subsequently analyzed by HPLC (Supplementary Methods). For the representative reaction: (i) control reaction lacking TDP-16; (ii) control reaction lacking GtfE; (iii) full reaction where 37 is donor, 60 is acceptor, 61e is desired product and ⋄ represents 2-chloro-4-nitrophenolate. Sample preparation and HPLC parameters, along with chromatograms (Supplementary Fig. 14 and 17), conversion rates, and mass characterization (Supplementary Table 4 and 5) for all products are presented in supporting online material.
Fig. 5
Utilizing a colorimetric screen for glycosyl transfer. (a) Scheme for colorimetric screen using the single enzyme (TDP-16) coupled format. (b) Evaluation of the colorimetric assay with 58 as the final acceptor. The reactions contained 0.5 mM 9 as donor, 0.5 mM 58 as acceptor, 5 μM UDP, and 11μM TDP-16 in a final total volume of 100 μl with Tris-HCl buffer (50 mM, pH 8.5) in a 96-well plate incubated at 25°C for one hour. (i) Qualitative color change after one hour for the full reaction (yellow square), a control lacking the final acceptor 58 (white circle), and a control lacking UDP (red triangle). (ii) Δ410 nm over one hour for the full reaction (yellow squares), a control lacking the final acceptor 58 (white circles), and a control reaction lacking UDP (red triangles). (iii) HPLC chromatograms of full reaction at 1, 5, and 60 min where 1 is desired product, 9 is the donor, 58 is the target aglycon and ⋄ represents 2-chloro-4-nitrophenolate. (c) The absorbance data and HPLC chromatograms of three representative hits [(i) 62 (genistein), (ii) 79 (tyrphostin), or (iii) 92 (ciprofloxacin)] from the broad 50 compound panel screen using the single enzyme (TDP-16) coupled format. In HPLC chromatograms 9 indicates donor; 62, 79 or 92 represent target aglycon; ⋄ indicates 2-chloro-4-nitrophenolate; and ● depicts glucosylated product(s). For the overall results of the 50 compound screen, additional representative absorbance plots and chromatograms, and combined HPLC and LC/MS characterization, see Supplementary Fig. 19–21 and Supplementary Table 6.
Comment in
- Glycobiology: Challenging reaction equilibria.
Field RA. Field RA. Nat Chem Biol. 2011 Sep 19;7(10):658-9. doi: 10.1038/nchembio.668. Nat Chem Biol. 2011. PMID: 21931313 No abstract available.
Similar articles
- Broadening the scope of glycosyltransferase-catalyzed sugar nucleotide synthesis.
Gantt RW, Peltier-Pain P, Singh S, Zhou M, Thorson JS. Gantt RW, et al. Proc Natl Acad Sci U S A. 2013 May 7;110(19):7648-53. doi: 10.1073/pnas.1220220110. Epub 2013 Apr 22. Proc Natl Acad Sci U S A. 2013. PMID: 23610417 Free PMC article. - High-throughput colorimetric assays for nucleotide sugar formation and glycosyl transfer.
Gantt RW, Thorson JS. Gantt RW, et al. Methods Enzymol. 2012;516:345-60. doi: 10.1016/B978-0-12-394291-3.00009-5. Methods Enzymol. 2012. PMID: 23034237 - A simple strategy for glycosyltransferase-catalyzed aminosugar nucleotide synthesis.
Zhang J, Singh S, Hughes RR, Zhou M, Sunkara M, Morris AJ, Thorson JS. Zhang J, et al. Chembiochem. 2014 Mar 21;15(5):647-52. doi: 10.1002/cbic.201300779. Chembiochem. 2014. PMID: 24677528 Free PMC article. - The Sweet Side of Plant-Specialized Metabolism.
Louveau T, Osbourn A. Louveau T, et al. Cold Spring Harb Perspect Biol. 2019 Dec 2;11(12):a034744. doi: 10.1101/cshperspect.a034744. Cold Spring Harb Perspect Biol. 2019. PMID: 31235546 Free PMC article. Review. - Atomistic insight into the catalytic mechanism of glycosyltransferases by combined quantum mechanics/molecular mechanics (QM/MM) methods.
Tvaroška I. Tvaroška I. Carbohydr Res. 2015 Feb 11;403:38-47. doi: 10.1016/j.carres.2014.06.017. Epub 2014 Jun 24. Carbohydr Res. 2015. PMID: 25060837 Review.
Cited by
- Cell-Free Synthetic Glycobiology: Designing and Engineering Glycomolecules Outside of Living Cells.
Jaroentomeechai T, Taw MN, Li M, Aquino A, Agashe N, Chung S, Jewett MC, DeLisa MP. Jaroentomeechai T, et al. Front Chem. 2020 Jul 29;8:645. doi: 10.3389/fchem.2020.00645. eCollection 2020. Front Chem. 2020. PMID: 32850660 Free PMC article. Review. - Binuclear copper(II) complexes discriminating epimeric glycosides and _α_- and _β_-glycosidic bonds in aqueous solution.
Striegler S, Fan QH, Rath NP. Striegler S, et al. J Catal. 2016 Jun;338:349-364. doi: 10.1016/j.jcat.2015.12.026. J Catal. 2016. PMID: 27667854 Free PMC article. - Leloir Glycosyltransferases in Applied Biocatalysis: A Multidisciplinary Approach.
Mestrom L, Przypis M, Kowalczykiewicz D, Pollender A, Kumpf A, Marsden SR, Bento I, Jarzębski AB, Szymańska K, Chruściel A, Tischler D, Schoevaart R, Hanefeld U, Hagedoorn PL. Mestrom L, et al. Int J Mol Sci. 2019 Oct 23;20(21):5263. doi: 10.3390/ijms20215263. Int J Mol Sci. 2019. PMID: 31652818 Free PMC article. Review. - Terfestatins B and C, New p-Terphenyl Glycosides Produced by Streptomyces sp. RM-5-8.
Wang X, Reynolds AR, Elshahawi SI, Shaaban KA, Ponomareva LV, Saunders MA, Elgumati IS, Zhang Y, Copley GC, Hower JC, Sunkara M, Morris AJ, Kharel MK, Van Lanen SG, Prendergast MA, Thorson JS. Wang X, et al. Org Lett. 2015 Jun 5;17(11):2796-9. doi: 10.1021/acs.orglett.5b01203. Epub 2015 May 11. Org Lett. 2015. PMID: 25961722 Free PMC article. - Photooxidation of thiosaccharides mediated by sensitizers in aerobic and environmentally friendly conditions.
Traverssi MG, Peñéñory AB, Varela O, Colomer JP. Traverssi MG, et al. RSC Adv. 2021 Mar 1;11(16):9262-9273. doi: 10.1039/d0ra09534f. eCollection 2021 Mar 1. RSC Adv. 2021. PMID: 35423421 Free PMC article.
References
- Varki A, et al., editors. Essentials of Glycobiology. 2. Cold Spring Harbor; New York: 2009. - PubMed
- Thibodeaux CJ, Melancon CE, III, Liu H. Unusual sugar biosynthesis and natural product glycodiversification. Nature. 2007;446:1008–1016. - PubMed
- Lairson LL, Wakarchuk WW, Withers SG. Alternative donor substrates for inverting and retaining glycosyltransferases. ChemComm. 2007:365–367. - PubMed
- Lougheed B, Ly HD, Wakarchuk WW, Withers SG. Glycosyl fluorides as substrates for nucleotide phosphosugar-dependent glycosyltransferases. J Biol Chem. 1999;274:37717–37722. - PubMed
- Zhang C, et al. Exploiting the reversibility of natural product glycosyltransferase-catalyzed reactions. Science. 2006;313:1291–1294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AI052218/AI/NIAID NIH HHS/United States
- R01 AI052218/AI/NIAID NIH HHS/United States
- AI52218/AI/NIAID NIH HHS/United States
- R01 AI052218-08/AI/NIAID NIH HHS/United States
- R01 AI052218-09/AI/NIAID NIH HHS/United States
- R01 AI052218-07/AI/NIAID NIH HHS/United States
- R01 AI052218-10/AI/NIAID NIH HHS/United States
- R01 AI052218-08S1/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources