A role for heterochromatin protein 1γ at human telomeres - PubMed (original) (raw)
A role for heterochromatin protein 1γ at human telomeres
Silvia Canudas et al. Genes Dev. 2011.
Abstract
Human telomere function is mediated by shelterin, a six-subunit complex that is required for telomere replication, protection, and cohesion. TIN2, the central component of shelterin, has binding sites to three subunits: TRF1, TRF2, and TPP1. Here we identify a fourth partner, heterochromatin protein 1γ (HP1γ), that binds to a conserved canonical HP1-binding motif, PXVXL, in the C-terminal domain of TIN2. We show that HP1γ localizes to telomeres in S phase, where it is required to establish/maintain cohesion. We further demonstrate that the HP1-binding site in TIN2 is required for sister telomere cohesion and can impact telomere length maintenance by telomerase. Remarkably, the PTVML HP1-binding site is embedded in the recently identified cluster of mutations in TIN2 that gives rise to dyskeratosis congenita (DC), an inherited bone marrow failure syndrome caused by defects in telomere maintenance. We show that DC-associated mutations in TIN2 abrogate binding to HP1γ and that DC patient cells are defective in sister telomere cohesion. Our data indicate a novel requirement for HP1γ in the establishment/maintenance of cohesion at human telomeres and, furthermore, may provide insight into the mechanism of pathogenesis in TIN2-mediated DC.
Figures
Figure 1.
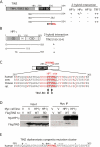
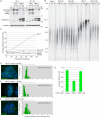
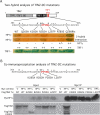
HP1 binds to the PTVML site in the DC-associated TIN2 mutation cluster. (A) Schematic representation of TIN2 and two-hybrid interaction with HP1. TPP1-, TRF2-, and TRF1-binding domains (BDs) and the double point mutation in TIN2 (RTDML) are indicated. (B) Schematic representation of HP1 and two-hybrid interaction with TIN2. The chromodomain (CD), hinge (H), and chromoshadow domain (CSD) in HP1 are indicated. (A,B) Two-hybrid interactions were scored according to the number of minutes required for the color change: 20 min (++), 45 min (+), and 150–180 min (+/−). (C) Alignment of the TIN2 domain containing the PTVML HP1-binding site. Identical amino acids are indicated in red. (D) HP1 binds to TIN2 in human cells. HT1080 stable cell lines expressing HP1γ or vector (V) were transfected with vector or FlagTIN2 (WT or RD). Cell lysates were immunoprecipitated with anti-myc beads and analyzed by immunoblotting with anti-myc or anti-TIN2 701 antibody. (E) The DC-associated mutation cluster in TIN2. DC mutations are indicated by dots and asterisks; asterisks indicate mutations shown to give rise to shortened telomeres.
Figure 2.
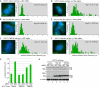
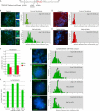
An intact HP1-binding site in TIN2 is required for sister telomere cohesion. (A–F) FISH analysis of HTC75 stable cell lines expressing Vector (A,D), TIN2.WT (B,E), or TIN2.RD (C,F) following transfection with GFP siRNA (A–C) or TIN2 siRNA (D–F) with a 16ptelo probe. DNA was stained with DAPI (blue). Bar, 5 μm. Histograms based on measurements from two independent experiments (Table 1) showing the distance between FISH signals are on the right, with the average (Avg) distance ± SEM indicated. (G) Graphical representation of the average distance between sister telomeres ± SEM. (H) Immunoblot analysis of extracts from stable HTC75 lines expressing Vector, TIN2.WT, or TIN2.RD transfected with GFP siRNA or TIN2 siRNA and probed with anti-TIN2 701 or anti-α-tubulin antibody. Asterisk (*) indicates a nonspecific band.
Figure 3.
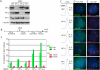
HP1 is required to establish/maintain sister telomere cohesion in S phase. (A) Immunoblot analysis of extracts from HeLaI.2.11 cells transfected for 48 h with GFP, HP1α, HP1β, or HP1γ siRNA. (B) Schematic representation of the protocol to analyze siRNA-treated synchronized cells. (C–E) FACS (C) and FISH (D,E) analysis 4 h after release from the second thymidine block. Cells were hybridized with a telomere 16ptelo (green) (D) or centromere 6cen (red) (E) probe. The cen locus is trisomic. DNA was stained with DAPI (blue). Bar, 5 μm. (F) Graphical representation of the frequency of doublets from two independent experiments: 16pter and 6cen probes (Experiment 1) and 4pter and 10cen probes (Experiment 2) (see Table 2).
Figure 4.
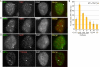
HP1γ colocalizes with TIN2 at telomeres in S phase. (A,C–E) Immunofluorescence analysis of HeLaI.2.11 cells 4 h after release from a double thymidine block. Cells were extracted with Triton prior to fixation and dually stained with antibodies against HP1γ (green) (A,C–E), TIN2 (red) (A), TRF1 (red) (C), and RAP1 (red) (D), or processed for FISH with a PNA-TTAGGG repeat probe (red) (E). Merge is yellow. Bar, 5 μm. (B) Graphical representation of the frequency of cells containing colocalizing HP1γ–TIN2 foci across the cell cycle. Cells were synchronized by a double thymidine block and released for 0 h (G1/S), 2 h (early S), 4 h (mid S), 6 h (late S), 8 h (G2), 10 h (M), and 12 h (G1). Mean ± SD of three independent experiments (n, ∼100 cells each).
Figure 5.
TIN2 mutations in the HP1-binding site interfere with telomere length maintenance. (A) Immunoblot analysis of extracts from stable HTC75 cell lines expressing Vector (V), TIN2.WT, TIN2.RD, TIN2-C, or TIN2-C.RD at population doubling 4 (PD 4) or 120 (PD 120) probed with anti-TIN2 701 or anti-α-tubulin antibody. (B) Analysis of telomere restriction fragments isolated from the stable HTC75 cell lines at the indicated population doubling, fractionated on agarose gel, denatured, and probed with a 32P-labeled CCCTAA probe. (C) Graphical representation of telomere length changes in B. (D–F) FISH analysis of HTC75 stable cell lines expressing Vector (D), TIN2-C (E), or TIN2-C.RD (F) with a 16ptelo probe. DNA was stained with DAPI (blue). Bar, 5 μm. Histograms based on measurements from two independent experiments (Table 1) showing the distance between FISH signals are on the right, with the average (Avg) distance ± SEM indicated. (G) Graphical representation of the average distance between sister telomeres ± SEM.
Figure 6.
DC-associated TIN2 mutations interfere with HP1 binding. (A) Two-hybrid analysis of the interaction between DC-associated TIN2 mutations and HP1γ and TRF1. Two-hybrid interactions were scored according to the number of minutes required for the color change: 20 min (++), 45 min (+), and 150–180 min (+/−). (B) Coimmunoprecipitation analysis of the interaction between DC-associated TIN2 mutations and HP1γ. HT1080 stable cell lines expressing HP1γ or vector (V) were transfected with the indicated FlagTIN2 plasmids. Cell lysates were immunoprecipitated with anti-myc beads and analyzed by immunoblotting with anti-myc or anti-TIN2 701 antibody.
Figure 7.
DC patient cells harboring TIN2 mutations suffer loss in sister telomere cohesion. (A) DC mutations in patient cell lines are indicated. (B–E) Loss in sister telomere cohesion in DC patient fibroblasts harboring the TIN2p.Q269X mutation. FISH analysis with a 16ptelo (B,C) or 10cen (D,E) probe. DNA was stained with DAPI (blue). Bar, 5 μm. Histograms showing the distance between FISH signals (Table 3) are on the right, with the average (Avg) distance ± SEM indicated. (F) Graphical representation of the average distance ± SEM. (G–K) Loss in sister telomere cohesion in DC patient LCLs harboring the following mutations: TIN2p.K280Rfs36X, TIN2p.R282H, and DKC1p.A2V. (G–J) FISH analysis with a 16ptelo probe. DNA was stained with DAPI (blue). Bar, 5 μm. Histograms showing the distance between FISH signals (Table 3) are shown on the right, with the average (Avg) distance ± SEM indicated. (K) Graphical representation of the average distance ± SEM.
Figure 8.
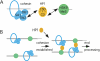
Schematic diagram showing how HP1 might influence cohesion at telomeres. (A) HP1 could act as a bridge in telomeric heterochromatin by binding to the heterochromatin mark H3K9Me3 via its CD and to TIN2-SA1 cohesin via its CSD. (B) HP1 associates with telomeres in S phase to aid in establishment of cohesion. Once cohesion is established, chromosome ends from both sisters could undergo coordinated processing, including C-strand resection, telomerase-dependent elongation, and C-strand synthesis.
Similar articles
- A role for sister telomere cohesion in telomere elongation by telomerase.
Houghtaling BR, Canudas S, Smith S. Houghtaling BR, et al. Cell Cycle. 2012 Jan 1;11(1):19-25. doi: 10.4161/cc.11.1.18633. Epub 2012 Jan 1. Cell Cycle. 2012. PMID: 22157096 Free PMC article. - TIN2 protein dyskeratosis congenita missense mutants are defective in association with telomerase.
Yang D, He Q, Kim H, Ma W, Songyang Z. Yang D, et al. J Biol Chem. 2011 Jul 1;286(26):23022-30. doi: 10.1074/jbc.M111.225870. Epub 2011 May 2. J Biol Chem. 2011. PMID: 21536674 Free PMC article. - The C-Terminal Extension Unique to the Long Isoform of the Shelterin Component TIN2 Enhances Its Interaction with TRF2 in a Phosphorylation- and Dyskeratosis Congenita Cluster-Dependent Fashion.
Nelson ND, Dodson LM, Escudero L, Sukumar AT, Williams CL, Mihalek I, Baldan A, Baird DM, Bertuch AA. Nelson ND, et al. Mol Cell Biol. 2018 May 29;38(12):e00025-18. doi: 10.1128/MCB.00025-18. Print 2018 Jun 15. Mol Cell Biol. 2018. PMID: 29581185 Free PMC article. - Short telomeres: from dyskeratosis congenita to sporadic aplastic anemia and malignancy.
Gramatges MM, Bertuch AA. Gramatges MM, et al. Transl Res. 2013 Dec;162(6):353-63. doi: 10.1016/j.trsl.2013.05.003. Epub 2013 Jun 1. Transl Res. 2013. PMID: 23732052 Free PMC article. Review. - Shelterin: the protein complex that shapes and safeguards human telomeres.
de Lange T. de Lange T. Genes Dev. 2005 Sep 15;19(18):2100-10. doi: 10.1101/gad.1346005. Genes Dev. 2005. PMID: 16166375 Review.
Cited by
- Discovery, expression, cellular localization, and molecular properties of a novel, alternative spliced HP1γ isoform, lacking the chromoshadow domain.
Mathison A, Milech De Assuncao T, Dsouza NR, Williams M, Zimmermann MT, Urrutia R, Lomberk G. Mathison A, et al. PLoS One. 2020 Feb 6;15(2):e0217452. doi: 10.1371/journal.pone.0217452. eCollection 2020. PLoS One. 2020. PMID: 32027651 Free PMC article. - TERRA recruitment of polycomb to telomeres is essential for histone trymethylation marks at telomeric heterochromatin.
Montero JJ, López-Silanes I, Megías D, F Fraga M, Castells-García Á, Blasco MA. Montero JJ, et al. Nat Commun. 2018 Apr 18;9(1):1548. doi: 10.1038/s41467-018-03916-3. Nat Commun. 2018. PMID: 29670078 Free PMC article. - Chromosome Healing Is Promoted by the Telomere Cap Component Hiphop in Drosophila.
Kurzhals RL, Fanti L, Ebsen ACG, Rong YS, Pimpinelli S, Golic KG. Kurzhals RL, et al. Genetics. 2017 Nov;207(3):949-959. doi: 10.1534/genetics.117.300317. Epub 2017 Sep 23. Genetics. 2017. PMID: 28942425 Free PMC article. - Telomeric G-quadruplexes are a substrate and site of localization for human telomerase.
Moye AL, Porter KC, Cohen SB, Phan T, Zyner KG, Sasaki N, Lovrecz GO, Beck JL, Bryan TM. Moye AL, et al. Nat Commun. 2015 Jul 9;6:7643. doi: 10.1038/ncomms8643. Nat Commun. 2015. PMID: 26158869 Free PMC article. - Obesity-Senescence-Breast Cancer: Clinical Presentation of a Common Unfortunate Cycle.
Engin AB, Engin A. Engin AB, et al. Adv Exp Med Biol. 2024;1460:821-850. doi: 10.1007/978-3-031-63657-8_27. Adv Exp Med Biol. 2024. PMID: 39287873 Review.
References
- Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, Kouzarides T 2001. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410: 120–124 - PubMed
- Bartel PL, Chien C, Sternglanz R, Fields S 1993. Using the two-hybrid system to detect protein–protein interaction. In Cellular interactions in development: a practical approach (ed. Harley DA), pp. 153–179 IRL Press, Oxford
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA116352/CA/NCI NIH HHS/United States
- N02CP65501/CP/NCI NIH HHS/United States
- N02CP11019/CP/NCI NIH HHS/United States
- N02CP65504/CP/NCI NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous