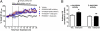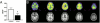Serotonin signaling is associated with lower amyloid-β levels and plaques in transgenic mice and humans - PubMed (original) (raw)
. 2011 Sep 6;108(36):14968-73.
doi: 10.1073/pnas.1107411108. Epub 2011 Aug 22.
Brianne M Disabato, Jessica L Restivo, Deborah K Verges, Whitney D Goebel, Anshul Sathyan, Davinder Hayreh, Gina D'Angelo, Tammie Benzinger, Hyejin Yoon, Jungsu Kim, John C Morris, Mark A Mintun, Yvette I Sheline
Affiliations
- PMID: 21873225
- PMCID: PMC3169155
- DOI: 10.1073/pnas.1107411108
Serotonin signaling is associated with lower amyloid-β levels and plaques in transgenic mice and humans
John R Cirrito et al. Proc Natl Acad Sci U S A. 2011.
Abstract
Aggregation of amyloid-β (Aβ) as toxic oligomers and amyloid plaques within the brain appears to be the pathogenic event that initiates Alzheimer's disease (AD) lesions. One therapeutic strategy has been to reduce Aβ levels to limit its accumulation. Activation of certain neurotransmitter receptors can regulate Aβ metabolism. We assessed the ability of serotonin signaling to alter brain Aβ levels and plaques in a mouse model of AD and in humans. In mice, brain interstitial fluid (ISF) Aβ levels were decreased by 25% following administration of several selective serotonin reuptake inhibitor (SSRI) antidepressant drugs. Similarly, direct infusion of serotonin into the hippocampus reduced ISF Aβ levels. Serotonin-dependent reductions in Aβ were reversed if mice were pretreated with inhibitors of the extracellular regulated kinase (ERK) signaling cascade. Chronic treatment with an SSRI, citalopram, caused a 50% reduction in brain plaque load in mice. To test whether serotonin signaling could impact Aβ plaques in humans, we retrospectively compared brain amyloid load in cognitively normal elderly participants who were exposed to antidepressant drugs within the past 5 y to participants who were not. Antidepressant-treated participants had significantly less amyloid load as quantified by positron emission tomography (PET) imaging with Pittsburgh Compound B (PIB). Cumulative time of antidepressant use within the 5-y period preceding the scan correlated with less plaque load. These data suggest that serotonin signaling was associated with less Aβ accumulation in cognitively normal individuals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
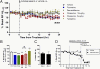
Fig. 1.
SSRIs reduce ISF Aβ levels in vivo. Two- to 3-mo-old PS1APP hemizygous mice were administered vehicle (PBS) or one of several antidepressants by i.p. injection: fluoxetine 10 mg/kg, desvenlafaxine 30 mg/kg, citalopram 5 mg/kg and 10 mg/kg, tianeptine 20 mg/kg (n = 5–8 per group). (A) As assessed by in vivo microdialysis, SSRIs reduced ISF Aβx-40 levels significantly beginning between 10 and 14 h after administration. (B) Twenty-four hours after administration, fluoxetine and desvenlafaxine reduced Aβx-40 levels to 73.4 ± 2.0% (P < 0.0001; n = 6) and 71.6 ± 1.2% (P = 0.001; n = 6), respectively, compared with baseline levels in each mouse. Doses of 10 mg/kg and 5 mg/kg citalopram reduced ISF Aβx-40 to 74.0 ± 5.4% (P = 0.004; n = 8) and 83.5 ± 4.2% (P = 0.02; n = 6) of baseline levels, respectively. ISF Aβ levels did not change significantly in tianeptine and vehicle-treated mice (n = 5 per group). (C) PS1APP mice treated with 10 mg/kg citalopram had similar reductions in ISF Aβx-40 and Aβx-42 levels; 71.9 ± 9.4% and 66.7 ± 16.3% of baseline levels, respectively, by 24 h posttreatment (n = 5). (D) PS1APP mice were treated with vehicle (artificial CSF, aCSF) or 2 mM serotonin directly to the hippocampus by reverse microdialysis for 14 h. By 8 h of administration, serotonin reduced ISF Aβx-40 levels to 66.7 ± 7.2% of baseline (P = 0.003, n = 5 per group). After 8 h, mice were administered a γ-secretase inhibitor, Compound E (20 mg/kg i.p.) to assess Aβx-40 half-life. Data presented as mean ± SEM.
Fig. 2.
ERK-dependent changes in Aβ metabolism. (A) Young PS1APP mice were administered vehicle (aCSF), a MEK inhibitor (PD98059, 100 μM), or an ERK inhibitor (FR180204, 100 μM) by reverse microdialysis (n = 6–8 per group). At 24 h from the beginning of treatment, the MEK and ERK inhibitors alone significantly increased ISF Aβ levels by 37.7 ± 3.9% (P = 0.001) and 39.4 ± 1.6% (P < 0.0001) compared with baseline levels. Coadministration of citalopram (10 mg/kg i.p.) with either of these inhibitors blocked the SSRI-dependent reduction in ISF Aβ. ISF Aβ levels were not significantly different between inhibitor-treated and inhibitor plus citalopram-treated mice. (B) Citalopram increased α-secretase enzymatic activity by 25 ± 4.9% (P = 0.01) within the hippocampus with no change in β-secretase activity (n = 8 per group). Data presented as mean ± SEM.
Fig. 3.
Chronic SSRI administration reduces plaque load in PS1APP transgenic mice. Beginning at 3 mo of age, PS1APP hemizygous mice were treated with water or citalopram (8 mg/kg/day) in drinking water for 4 mo (n = 10 per group). Representative images of cortex and hippocampus stained for Aβ plaques in (A) water and (B) citalopram-treated mice. (C) Quantification of plaque load in the hippocampus and cortex was performed blinded. Surface area covered by plaques was reduced in citalopram-treated mice to 57.9 ± 6.1% (P = 0.03) and 49.5 ± 6.2% (P = 0.004) of mean levels in water-treated mice. (D) Guanidine-extracted Aβx-40 and Aβx-42 in the hippocampus and cortex was significantly reduced in citalopram-treated mice. (E) Aβx-40 and Aβx-42 levels within the CSF of citalopram-treated mice were reduced to 71.4 ± 7.5% (P = 0.02) and 49.5 ± 6.2% (P < 0.0001) compared with mean levels in water-treated mice. (F) α-Secretase enzymatic activity was significantly increased in chronic citalopram mice (P < 0.001); however, β-secretase activity was unchanged. (G) Quantitative PCR showed that mRNA levels of ADAM10 did not change significantly but that memapsin-2 (P = 0.01) and two components of the γ-secretase complex, presenilin-1 and nicastrin, were significantly reduced in citalopram-treated mice (P = 0.02 and P = 0.01; n = 9–10 per group). APH-1B, neprilysin, and LRP1 mRNA levels did not change following citalopram treatment. Values normalized to mean level in water-treated mice. Data presented as mean ± SEM.
Fig. 4.
Antidepressant use is associated with less cortical amyloid in human participants. (A) Mean cortical binding potential of PIB (MCBP) in participants who had taken antidepressants within the past 5 y “treated” versus those who had not been treated “untreated.” Mean exposure time for the treated group = 34.5 mo (SD 23.6). MCBP in untreated participants was 0.13 ± 0.22 (n = 134; mean ± SD) versus 0.06 ± 0.20 (n = 43; P = 0.01). (B) Normalized Aβ PET subtraction image of untreated participants, minus treated participants, showing the pattern of cortical rim Aβ plaque accumulation typical of AD.
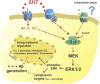
Fig. 5.
Model of serotonergic regulation of Aβ metabolism. Serotonin receptors are activated on the cell surface, which initiates a signaling cascade that leads to ERK phosphorylation and activation. ERK appears to increase α-secretase cleavage and may reduce γ-secretase cleavage of APP. The particular serotonin receptor subtypes responsible for ERK activation in this paradigm are unknown. Not all receptors or ligands that activate ERK will alter APP processing, however similar to serotonin, NMDA receptors can also modulate APP processing through the ERK pathway.
Comment in
- Potential effects of the APOE epsilon2 allele and of family history of Alzheimer's disease on brain amyloid-β in normal elderly.
Pomara N, Bruno D. Pomara N, et al. Proc Natl Acad Sci U S A. 2011 Nov 8;108(45):E1007; author reply E1008. doi: 10.1073/pnas.1114219108. Epub 2011 Oct 31. Proc Natl Acad Sci U S A. 2011. PMID: 22042836 Free PMC article. No abstract available.
Similar articles
- An antidepressant decreases CSF Aβ production in healthy individuals and in transgenic AD mice.
Sheline YI, West T, Yarasheski K, Swarm R, Jasielec MS, Fisher JR, Ficker WD, Yan P, Xiong C, Frederiksen C, Grzelak MV, Chott R, Bateman RJ, Morris JC, Mintun MA, Lee JM, Cirrito JR. Sheline YI, et al. Sci Transl Med. 2014 May 14;6(236):236re4. doi: 10.1126/scitranslmed.3008169. Sci Transl Med. 2014. PMID: 24828079 Free PMC article. Clinical Trial. - Serotonin augmentation therapy by escitalopram has minimal effects on amyloid-β levels in early-stage Alzheimer's-like disease in mice.
von Linstow CU, Waider J, Grebing M, Metaxas A, Lesch KP, Finsen B. von Linstow CU, et al. Alzheimers Res Ther. 2017 Sep 12;9(1):74. doi: 10.1186/s13195-017-0298-y. Alzheimers Res Ther. 2017. PMID: 28899417 Free PMC article. - Effect of escitalopram on Aβ levels and plaque load in an Alzheimer mouse model.
Cirrito JR, Wallace CE, Yan P, Davis TA, Gardiner WD, Doherty BM, King D, Yuede CM, Lee JM, Sheline YI. Cirrito JR, et al. Neurology. 2020 Nov 10;95(19):e2666-e2674. doi: 10.1212/WNL.0000000000010733. Epub 2020 Sep 10. Neurology. 2020. PMID: 32913022 Free PMC article. - [Molecular imaging of beta-amyloid plaques in the brain].
Ono M. Ono M. Brain Nerve. 2007 Mar;59(3):233-40. Brain Nerve. 2007. PMID: 17370649 Review. Japanese. - Development and evaluation of compounds for imaging of beta-amyloid plaque by means of positron emission tomography.
Henriksen G, Yousefi BH, Drzezga A, Wester HJ. Henriksen G, et al. Eur J Nucl Med Mol Imaging. 2008 Mar;35 Suppl 1:S75-81. doi: 10.1007/s00259-007-0705-x. Eur J Nucl Med Mol Imaging. 2008. PMID: 18224319 Review.
Cited by
- HTR3A is correlated with unfavorable histology and promotes proliferation through ERK phosphorylation in lung adenocarcinoma.
Tone M, Tahara S, Nojima S, Motooka D, Okuzaki D, Morii E. Tone M, et al. Cancer Sci. 2020 Oct;111(10):3953-3961. doi: 10.1111/cas.14592. Epub 2020 Aug 17. Cancer Sci. 2020. PMID: 32736413 Free PMC article. - Association of Midlife Depressive Symptoms with Regional Amyloid-β and Tau in the Framingham Heart Study.
Gonzales MM, Samra J, O'Donnell A, Mackin RS, Salinas J, Jacob ME, Satizabal CL, Aparicio HJ, Thibault EG, Sanchez JS, Finney R, Rubinstein ZB, Mayblyum DV, Killiany RJ, Decarli CS, Johnson KA, Beiser AS, Seshadri S. Gonzales MM, et al. J Alzheimers Dis. 2021;82(1):249-260. doi: 10.3233/JAD-210232. J Alzheimers Dis. 2021. PMID: 34024836 Free PMC article. - Intestinal Flora Affect Alzheimer's Disease by Regulating Endogenous Hormones.
Wu Y, Hang Z, Lei T, Du H. Wu Y, et al. Neurochem Res. 2022 Dec;47(12):3565-3582. doi: 10.1007/s11064-022-03784-w. Epub 2022 Oct 30. Neurochem Res. 2022. PMID: 36309938 Review. - Single-cell RNA-sequencing identifies disease-associated oligodendrocytes in male APP NL-G-F and 5XFAD mice.
Park H, Cho B, Kim H, Saito T, Saido TC, Won KJ, Kim J. Park H, et al. Nat Commun. 2023 Feb 13;14(1):802. doi: 10.1038/s41467-023-36519-8. Nat Commun. 2023. PMID: 36781874 Free PMC article. - The amyloid precursor protein: a converging point in Alzheimer's disease.
Delport A, Hewer R. Delport A, et al. Mol Neurobiol. 2022 Jul;59(7):4501-4516. doi: 10.1007/s12035-022-02863-x. Epub 2022 May 17. Mol Neurobiol. 2022. PMID: 35579846 Review.
References
- St George-Hyslop PH, Morris JC. Will anti-amyloid therapies work for Alzheimer's disease? Lancet. 2008;372:180–182. - PubMed
- Morris JC, Price AL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer's disease. J Mol Neurosci. 2001;17:101–118. - PubMed
- Cirrito JR, et al. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K24 MH079510/MH/NIMH NIH HHS/United States
- P01 AG03991/AG/NIA NIH HHS/United States
- R21 MH77124/MH/NIMH NIH HHS/United States
- P30 NS069329/NS/NINDS NIH HHS/United States
- R21 MH077124/MH/NIMH NIH HHS/United States
- K24 MHO79510/PHS HHS/United States
- P50 AG05681/AG/NIA NIH HHS/United States
- K01 AG029524/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous