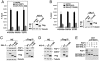Human Polymerase-Associated Factor complex (PAFc) connects the Super Elongation Complex (SEC) to RNA polymerase II on chromatin - PubMed (original) (raw)
Human Polymerase-Associated Factor complex (PAFc) connects the Super Elongation Complex (SEC) to RNA polymerase II on chromatin
Nanhai He et al. Proc Natl Acad Sci U S A. 2011.
Abstract
The Super Elongation Complex (SEC), containing transcription elongation activators/coactivators P-TEFb, ELL2, AFF4/1, ENL, and AF9, is recruited by HIV-1 Tat and mixed lineage leukemia (MLL) proteins to activate the expression of HIV-1 and MLL-target genes, respectively. In the absence of Tat and MLL, however, it is unclear how SEC is targeted to RNA polymerase (Pol) II to stimulate elongation in general. Furthermore, although ENL and AF9 can bind the H3K79 methyltransferase Dot1L, it is unclear whether these bindings are required for SEC-mediated transcription. Here, we show that the homologous ENL and AF9 exist in separate SECs with similar but nonidentical functions. ENL/AF9 contacts the scaffolding protein AFF4 that uses separate domains to recruit different subunits into SEC. ENL/AF9 also exists outside SEC when bound to Dot1L, which is found to inhibit SEC function. The YEATS domain of ENL/AF9 targets SEC to Pol II on chromatin through contacting the human Polymerase-Associated Factor complex (PAFc) complex. This finding explains the YEATS domain's dispensability for leukemogenesis when ENL/AF9 is translocated to MLL, whose interactions with PAFc and DNA likely substitute for the PAFc/chromatin-targeting function of the YEATS domain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
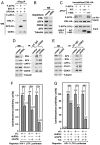
Fig. 1.
The scaffolding protein AFF4 directly binds to the C-terminal regions of ENL and AF9 to mediate their interactions with P-TEFb. (A). NE were prepared from HeLa cells either containing an empty vector or expressing the AFF4-specifc shRNA (shAFF4) and subjected to immunoprecipitation (IP) with anti-CDK9 or an irrelevant rabbit IgG as a control. The isolated NE (left box) and immunoprecipitates (right box) were analyzed by Western blotting with the indicated antibodies. (B). The indicated highly purified proteins were incubated with immobilized CycT1-HA/CDK9 in vitro and the bound proteins were eluted and analyzed by Western blotting (right). Five percent of the input proteins were also examined by anti-Flag Western blotting (left). (C). In vitro binding reactions were performed by incubating highly purified WT or C-terminally truncated ENL-F or AF9-F (schematic diagram on the right) with immobilized HA-AFF4. The bound proteins and 10% of the soluble input proteins were analyzed by Western blotting with the indicated antibodies. (D). WT or mutant ENL and AF9, all Flag-tagged, were expressed in transfected HeLa cells. Anti-Flag immunoprecipitates were examined by Western blotting for the indicated ENL/AF9-associated factors.
Fig. 2.
Separate regions of AFF4 are used to interact with different subunits of SEC. NEs derived from HeLa cells, which were transfected with cDNA constructs expressing either WT Flag-tagged AFF or the various deletion mutants as indicated, were subjected to anti-Flag IP. The immunoprecipitates were analyzed by Western blotting with the indicated antibodies. The diagram at the bottom summarizes the findings of the binding study.
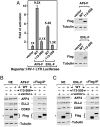
Fig. 3.
ENL and AF9 do not exist in the same SEC complex and exert similar but nonidentical functions in supporting SEC-dependent HIV-1 transcription. (A). Anti-Flag immunoprecipitates were obtained from NE of cells transfected with the various cDNA constructs and analyzed by Western blotting with the indicated antibodies. (B). HeLa NE were subjected to immunodepletion to remove ENL and the depleted NE were analyzed for the presence of the indicated proteins by Western blotting. (C). In vitro binding reactions contained constant amounts of ENL-HA immobilized on anti-HA beads and F-AFF4 in solution. WT or C-terminally deleted AF9-F were either not added (−) or added (+) in 3-fold increments into the binding reactions. The bound and input proteins were examined by Western blotting as indicated. (D and E). NE from HeLa cells expressing either shENL (D) or shAF9 (E) were subjected to IP with anti-CDK9 or an irrelevant rabbit IgG. The isolated NE (left) and immunoprecipitates (right) were analyzed by Western blotting with the indicated antibodies. (F and G). HeLa cells containing an integrated HIV-1 LTR-luciferase reporter gene were transfected with the Tat cDNA and/or plasmids expressing shENL, shAF9, or an irrelevant control sequence (ctl.). Luciferase activities were measured in cell extracts. The basal LTR activity in F and Tat-activated LTR activity in G were artificially set to “1” and “100”, respectively, for easy comparison. The error bars represent mean + /-SD.
Fig. 4.
Dot1L competes with AFF4 for binding to ENL/AF9 and does not exist in SEC. (A and B). HeLa cells were transfected with the indicated cDNA constructs. NE (left boxes) and immunoprecipitates isolated from NE (right boxes) with either anti-Flag (A) or anti-HA beads (B) were examined by Western blotting for the presence of the indicated proteins. (C and D). In vitro binding assay was performed in reactions containing HA-AFF4 immobilized on anti-HA beads, WT, or the C-terminally truncated AF9 or ENL, and different amounts of Dot1L. The bound and input proteins were examined by Western blotting as indicated. (E). HeLa cells containing a copy of integrated HIV-1 LTR-luciferase reporter construct were transfected with Dot1L-specific or control scrambled siRNA (ctl.). After six days of transfection, cells were transduced with a retrovirus encoding for Tat or empty vector. Luciferase was measured 24 h post transduction. Fold activation is the ratio between luciferase values obtained in the presence or absence of Tat for each siRNA. The graph represents mean and standard error obtained from three independent experiments. (F). Knock-down efficiency of siRNA treatment was measured by Western blot with specific antibodies as indicated.
Fig. 5.
The YEATS domains of ENL and AF9 are not required for SEC formation but essential for SEC-dependent HIV-1 transcription. (A). HeLa cells containing an integrated HIV-1 LTR-luciferase reporter gene were transfected with the indicated expression constructs. Left box: luciferase activities were measured in cell extracts, with the activity in cells transfected with an empty vector artificially set to “1.” The error bars represent mean + /-SD. Right boxes: Western analysis of the levels of WT and N-terminally deleted AF9-F and ENL-F in transfected cells. (B and C). HeLa cells were transfected with the indicated cDNA constructs. NE (left boxes) and anti-Flag immunoprecipitates isolated from NE (right boxes) were examined by Western blotting for the presence of the indicated proteins. A nonspecific band is indicated by asterisks (*).
Fig. 6.
ENL/AF9 YEATS domain interacts directly with PAF1 to target SEC to a chromatin template. (A and B). ChIP with the anti-Flag antibody was performed in cells containing an integrated HIV-1 LTR-luciferase reporter gene and transiently expressing WT or N-terminally deleted AF9-F (A) or ENL-F (B). Three regions corresponding to the promoter, interior, and 3′ UTR of the integrated reporter gene were qPCR-amplified from the precipitated and purified DNA and shown as percentages of the input chromatin. The error bars represent mean + /-SD. The levels of WT and mutant AF9-F or ENL-F in NE were examined by anti-Flag Western blotting in the right boxes. (C and D). HeLa cells were transfected with the indicated ENL-F (C) or AF9-F-expressing constructs (D). NE (left boxes) and anti-Flag immunoprecipitates isolated from NE (right boxes) were examined by Western blotting for the presence of the indicated proteins. A nonspecific band in (D) is indicated by an asterisk (*). (E). The GST pull-down assay was performed with the indicated proteins present in the reactions. After extensive washing, the proteins bound to the GST beads were detected by silver staining. GST-ENL-N and GST-ENL-C contain amino acids 1-154 and 433-559 of ENL, respectively.
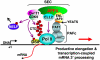
Fig. 7.
PAFc connects SEC to Pol II. (A). HeLa cells were transfected with the indicated ENL-F-expressing constructs. NE (left boxes) and anti-Flag immunoprecipitates isolated from NE (right boxes) were examined by Western blotting for the presence of the indicated proteins. (B and C). NE from HeLa cells either containing an empty vector or expressing the indicated shRNAs (B) or from the inducible shPAF1-expressing cells treated with (+) or without (−) doxycycline (Dox) to induce shPAF1 expression (C) were subjected to IP with the indicated antibodies. The isolated NE (left) and immunoprecipitates (right) were analyzed by Western blotting with the indicated antibodies. (D). The ChIP assay was performed in HeLa cells with either the anti-CDK9 or an irrelevant control antibody. Interior regions of the c-Myc and HEXIM1 gene were amplified by qPCR from the precipitated and purified DNA and shown as percentages of the input chromatin. The error bars represent mean + /-SD. E. A model showing the recruitment of the SEC complex, which contains either ENL or AF9 and is assembled around the scaffolding protein AFF4, to the elongating Pol II through the interaction of the ENL/AF9 YEATS domain with the PAF1 subunit of PAFc. This configuration allows SEC to use its P-TEFb and ELL2 functional modules to exert a multitude of effects that include the phosphorylation of the Pol II CTD and elongation factors DSIF and NELF (the latter is released upon phosphorylation) by CDK9 and the suppression of Pol II pausing by ELL2. These events synergistically activate productive elongation and likely also transcription-coupled mRNA 3′ processing.
Fig. P1.
Recruitment of the Super Elongation Complex, which contains either ENL or AF9 and is assembled around the scaffolding protein AFF4, to the elongating Pol II through the interaction of the ENL/AF9 YEATS domain with the PAF1 subunit of PAFc. This configuration allows the Super Elongation Complex to use its P-TEFb and ELL2 functional modules to exert a multitude of effects that result in the synergistic activation of Pol II elongation and mRNA 3′ end formation.
Similar articles
- Collaboration of MLLT1/ENL, Polycomb and ATM for transcription and genome integrity.
Ui A, Yasui A. Ui A, et al. Nucleus. 2016 Apr 25;7(2):138-45. doi: 10.1080/19491034.2016.1177681. Nucleus. 2016. PMID: 27310306 Free PMC article. Review. - Super elongation complex promotes early HIV transcription and its function is modulated by P-TEFb.
Kuzmina A, Krasnopolsky S, Taube R. Kuzmina A, et al. Transcription. 2017 May 27;8(3):133-149. doi: 10.1080/21541264.2017.1295831. Epub 2017 Feb 17. Transcription. 2017. PMID: 28340332 Free PMC article. - Small-molecule inhibitor of AF9/ENL-DOT1L/AF4/AFF4 interactions suppresses malignant gene expression and tumor growth.
Wu F, Nie S, Yao Y, Huo T, Li X, Wu X, Zhao J, Lin YL, Zhang Y, Mo Q, Song Y. Wu F, et al. Theranostics. 2021 Jul 13;11(17):8172-8184. doi: 10.7150/thno.56737. eCollection 2021. Theranostics. 2021. PMID: 34373735 Free PMC article. - HIV-1 Tat and host AFF4 recruit two transcription elongation factors into a bifunctional complex for coordinated activation of HIV-1 transcription.
He N, Liu M, Hsu J, Xue Y, Chou S, Burlingame A, Krogan NJ, Alber T, Zhou Q. He N, et al. Mol Cell. 2010 May 14;38(3):428-38. doi: 10.1016/j.molcel.2010.04.013. Mol Cell. 2010. PMID: 20471948 Free PMC article. - The super elongation complex (SEC) family in transcriptional control.
Luo Z, Lin C, Shilatifard A. Luo Z, et al. Nat Rev Mol Cell Biol. 2012 Sep;13(9):543-7. doi: 10.1038/nrm3417. Epub 2012 Aug 16. Nat Rev Mol Cell Biol. 2012. PMID: 22895430 Review.
Cited by
- Collaboration of MLLT1/ENL, Polycomb and ATM for transcription and genome integrity.
Ui A, Yasui A. Ui A, et al. Nucleus. 2016 Apr 25;7(2):138-45. doi: 10.1080/19491034.2016.1177681. Nucleus. 2016. PMID: 27310306 Free PMC article. Review. - The mechanism of MYB transcriptional regulation by MLL-AF9 oncoprotein.
Cao L, Mitra P, Gonda TJ. Cao L, et al. Sci Rep. 2019 Dec 27;9(1):20084. doi: 10.1038/s41598-019-56426-7. Sci Rep. 2019. PMID: 31882723 Free PMC article. - Short chain fatty acids potently induce latent HIV-1 in T-cells by activating P-TEFb and multiple histone modifications.
Das B, Dobrowolski C, Shahir AM, Feng Z, Yu X, Sha J, Bissada NF, Weinberg A, Karn J, Ye F. Das B, et al. Virology. 2015 Jan 1;474:65-81. doi: 10.1016/j.virol.2014.10.033. Epub 2014 Nov 14. Virology. 2015. PMID: 25463605 Free PMC article. - Post-translational modification-dependent oligomerization switch in regulation of global transcription and DNA damage repair during genotoxic stress.
Talukdar P, Pal S, Biswas D. Talukdar P, et al. Nat Commun. 2024 May 15;15(1):4128. doi: 10.1038/s41467-024-48530-8. Nat Commun. 2024. PMID: 38750015 Free PMC article. - MLL-Rearranged Leukemias-An Update on Science and Clinical Approaches.
Winters AC, Bernt KM. Winters AC, et al. Front Pediatr. 2017 Feb 9;5:4. doi: 10.3389/fped.2017.00004. eCollection 2017. Front Pediatr. 2017. PMID: 28232907 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- P50 GM082250-04/GM/NIGMS NIH HHS/United States
- R01AI095057/AI/NIAID NIH HHS/United States
- R01 AI095057/AI/NIAID NIH HHS/United States
- R01 AI041757/AI/NIAID NIH HHS/United States
- P50 GM082250/GM/NIGMS NIH HHS/United States
- R01AI41757/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical