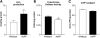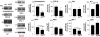Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer's disease - PubMed (original) (raw)
. 2011 Dec 1;20(23):4515-29.
doi: 10.1093/hmg/ddr381. Epub 2011 Aug 25.
Affiliations
- PMID: 21873260
- PMCID: PMC3209824
- DOI: 10.1093/hmg/ddr381
Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer's disease
Marcus J Calkins et al. Hum Mol Genet. 2011.
Abstract
Increasing evidence suggests that the accumulation of amyloid beta (Aβ) in synapses and synaptic mitochondria causes synaptic mitochondrial failure and synaptic degeneration in Alzheimer's disease (AD). The purpose of this study was to better understand the effects of Aβ in mitochondrial activity and synaptic alterations in neurons from a mouse model of AD. Using primary neurons from a well-characterized Aβ precursor protein transgenic (AβPP) mouse model (Tg2576 mouse line), for the first time, we studied mitochondrial activity, including axonal transport of mitochondria, mitochondrial dynamics, morphology and function. Further, we also studied the nature of Aβ-induced synaptic alterations, and cell death in primary neurons from Tg2576 mice, and we sought to determine whether the mitochondria-targeted antioxidant SS31 could mitigate the effects of oligomeric Aβ. We found significantly decreased anterograde mitochondrial movement, increased mitochondrial fission and decreased fusion, abnormal mitochondrial and synaptic proteins and defective mitochondrial function in primary neurons from AβPP mice compared with wild-type (WT) neurons. Transmission electron microscopy revealed a large number of small mitochondria and structurally damaged mitochondria, with broken cristae in AβPP primary neurons. We also found an increased accumulation of oligomeric Aβ and increased apoptotic neuronal death in the primary neurons from the AβPP mice relative to the WT neurons. Our results revealed an accumulation of intraneuronal oligomeric Aβ, leading to mitochondrial and synaptic deficiencies, and ultimately causing neurodegeneration in AβPP cultures. However, we found that the mitochondria-targeted antioxidant SS31 restored mitochondrial transport and synaptic viability, and decreased the percentage of defective mitochondria, indicating that SS31 protects mitochondria and synapses from Aβ toxicity.
Figures
Figure 1.
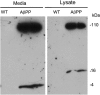
Four-kiloDalton Aβ is secreted into the media of AβPP-derived neurons. Twenty-five micrograms of the cell lysate or 10 µl of conditioned cell media from WT and AβPP cultures (16 DIV) was resolved on a 10–20% tricine gel, and AβPP and Aβ proteins were detected using AβPP- and Aβ-specific antibody (6E10, which recognizes Aβ residues 1–16) after following standard immunoblotting procedure. Left panel, media from the AβPP and WT cultures show an accumulation of 4 kDa Aβ monomer and 100 kDa-secreted AβPP. Right panel, cell lysates from AβPP and WT cultures show an accumulation of 110 kDa full-length AβPP and 16 kDa Aβ oligomer.
Figure 2.
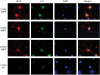
Aβ oligomer accumulates in cell neurites and soma over time. Double-immunocytochemistry with the A11 (anti-oligomer: green) and 6E10 (red) antibodies revealed 6E10 immunoreactivity present at all time points examined (10, 16, 22 DIV), indicated by increased staining in the cell body by 22 DIV. A11 immunoreactivity was not seen in appreciable amounts at 10 DIV, but was found extensively throughout neurites at 16 DIV. By 22 DIV, A11 staining was found within the cell body. WT neurons did not exhibit specific staining with the 6E10 or A11 antibodies. Images were all collected with a 100× objective.
Figure 3.
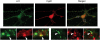
A11 immunoreactivity colocalizes with cyclophilin D staining. Double-labeling with A11 (green) and cyclophilin D (red) showed that Aβ oligomers colocalize with a mitochondrial matrix marker (cyclophilin D). Lower panels are enlargements of insets on the original images. Images were taken with a 100× objective. Arrowheads indicate colocalization.
Figure 4.
Primary neurons from AβPP mice undergo premature apoptotic cell death compared with WT neurons. (A) Cells stained with the Apo-ssDNA antibody for apoptosis were counted and normalized to DAPI-stained nuclei in order to assess the percentage of apoptotic nuclei (n = 5 cultures from independent pups per group). (B) Apo-ssDNA-stained cells colocalized with pyknotic nuclei from DAPI stain. (C) Representative images of Apo-ssDNA-stained cultures from WT and AβPP-derived cultures harvested at 10, 16 and 22 DIV.
Figure 5.
Mitochondrial function is impaired in AβPP cultures. (A) The content of hydrogen peroxide increased in AβPP compared with that in WT neurons. n = 4 independent cultures per group. (B) Cytochrome c oxidase activity decreased in AβPP neurons compared with WT. n = 6 independent cultures per group. (C) ATP levels were slightly decreased in the AβPP cultures compared with the ATP levels in the WT cultures. n = 4 independent cultures per group.
Figure 6.
Western blot analysis of AβPP cultures shows reduced expression of proteins involved in synaptic formation and mitochondrial fusion, but increased expression of the mitochondrial fission protein Drp1. Protein samples were probed for synaptophysin, PSD-95, Drp1, Fis1, Mfn1, Mfn2, OPA-1, cyclophilin D and β-actin. Representative blots are shown with quantification. n = 3 cultures per group. *P ≤ 0.05, **P ≤ 0.01 compared with WT.
Figure 7.
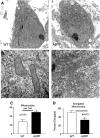
Transmission electron microscopy analysis of cell body mitochondria shows increased numbers of rounded mitochondria in AβPP neurons. (A) Representative images of cell bodies are shown from AβPP-derived and WT primary neuronal cultures at 16 DIV. (B) Close examination reveals that many mitochondria in AβPP neurons lack clear membrane structure and appear fragmented. (C) Mitochondria per cell body were counted. n ≥ 10 images per group. (D) Elongated mitochondria were counted and normalized to total mitochondria to calculate the percent of elongated mitochondria. n ≥ 10 images per group.
Figure 8.
Primary neurons from AβPP mice show reduced anterograde axonal transport of mitochondria but are rescued by mitochondria-targeted antioxidant, SS31. (A) Percent of motile mitochondria in neuronal cultures of AβPP, AβPP+SS31, WT, and WT+SS31. (B) Mitochondrial speed in all four neuronal cultures. (C) Anterograde movements of mitochondria in neuronal cultures of AβPP, AβPP+SS31, WT and WT+SS31. *P < 0.05; **P < 0.005.
Figure 9.
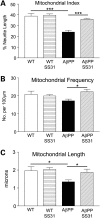
Mitochondrial index, frequency and length were reduced in primary neurons from AβPP mice. (A) Mitochondrial index, (B) mitochondrial frequency and (C) mitochondrial length in neuronal cultures of AβPP, AβPP+SS31, WT, and WT+SS31. *P < 0.05; **P < 0.005.
Similar articles
- Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer's disease neurons.
Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B, Reddy PH. Manczak M, et al. J Alzheimers Dis. 2010;20 Suppl 2(Suppl 2):S609-31. doi: 10.3233/JAD-2010-100564. J Alzheimers Dis. 2010. PMID: 20463406 Free PMC article. - Amyloid beta impairs mitochondrial anterograde transport and degenerates synapses in Alzheimer's disease neurons.
Calkins MJ, Reddy PH. Calkins MJ, et al. Biochim Biophys Acta. 2011 Apr;1812(4):507-13. doi: 10.1016/j.bbadis.2011.01.007. Epub 2011 Jan 15. Biochim Biophys Acta. 2011. PMID: 21241801 Free PMC article. - Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer's disease: implications to mitochondria-targeted antioxidant therapeutics.
Reddy PH, Tripathi R, Troung Q, Tirumala K, Reddy TP, Anekonda V, Shirendeb UP, Calkins MJ, Reddy AP, Mao P, Manczak M. Reddy PH, et al. Biochim Biophys Acta. 2012 May;1822(5):639-49. doi: 10.1016/j.bbadis.2011.10.011. Epub 2011 Oct 19. Biochim Biophys Acta. 2012. PMID: 22037588 Free PMC article. Review. - Cyclophilin D deficiency rescues axonal mitochondrial transport in Alzheimer's neurons.
Guo L, Du H, Yan S, Wu X, McKhann GM, Chen JX, Yan SS. Guo L, et al. PLoS One. 2013;8(1):e54914. doi: 10.1371/journal.pone.0054914. Epub 2013 Jan 31. PLoS One. 2013. PMID: 23382999 Free PMC article. - Abnormal tau, mitochondrial dysfunction, impaired axonal transport of mitochondria, and synaptic deprivation in Alzheimer's disease.
Reddy PH. Reddy PH. Brain Res. 2011 Sep 30;1415:136-48. doi: 10.1016/j.brainres.2011.07.052. Epub 2011 Jul 31. Brain Res. 2011. PMID: 21872849 Free PMC article. Review.
Cited by
- Rab11 suppresses neuronal stress signaling by localizing dual leucine zipper kinase to axon terminals for protein turnover.
Kim SM, Quagraine Y, Singh M, Kim JH. Kim SM, et al. Elife. 2024 Oct 30;13:RP96592. doi: 10.7554/eLife.96592. Elife. 2024. PMID: 39475475 Free PMC article. - Withania somnifera (Ashwagandha) Improves Spatial Memory, Anxiety and Depressive-like Behavior in the 5xFAD Mouse Model of Alzheimer's Disease.
Gladen-Kolarsky N, Monestime O, Bollen M, Choi J, Yang L, Magaña AA, Maier CS, Soumyanath A, Gray NE. Gladen-Kolarsky N, et al. Antioxidants (Basel). 2024 Sep 25;13(10):1164. doi: 10.3390/antiox13101164. Antioxidants (Basel). 2024. PMID: 39456417 Free PMC article. - Decoding Cancer through Silencing the Mitochondrial Gatekeeper VDAC1.
Arif T, Shteinfer-Kuzmine A, Shoshan-Barmatz V. Arif T, et al. Biomolecules. 2024 Oct 15;14(10):1304. doi: 10.3390/biom14101304. Biomolecules. 2024. PMID: 39456237 Free PMC article. Review. - Alzheimer's Disease Pathology and Assistive Nanotheranostic Approaches for Its Therapeutic Interventions.
Dey A, Ghosh S, Rajendran RL, Bhuniya T, Das P, Bhattacharjee B, Das S, Mahajan AA, Samant A, Krishnan A, Ahn BC, Gangadaran P. Dey A, et al. Int J Mol Sci. 2024 Sep 7;25(17):9690. doi: 10.3390/ijms25179690. Int J Mol Sci. 2024. PMID: 39273645 Free PMC article. Review.
References
- Selkoe D.J. Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. - PubMed
- LaFerla F.M., Green K.N., Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat. Rev. Neurosci. 2007;8:499–509. - PubMed
- Keller J.N., Pang Z., Geddes J.W., Begley J.G., Germeyer A., Waeg G., Mattson M.P. Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: role of the lipid peroxidation product 4-hydroxynonenal. J. Neurochem. 1997;69:273–284. - PubMed
- Swerdlow R.H., Khan S.M.A. Mitochondrial cascade hypothesis” for sporadic Alzheimer's disease. Med. Hypotheses. 2004;63:8–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases