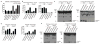Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity - PubMed (original) (raw)
Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity
Eric M Kofoed et al. Nature. 2011.
Abstract
Inflammasomes are a family of cytosolic multiprotein complexes that initiate innate immune responses to pathogenic microbes by activating the caspase 1 protease. Although genetic data support a critical role for inflammasomes in immune defence and inflammatory diseases, the molecular basis by which individual inflammasomes respond to specific stimuli remains poorly understood. The inflammasome that contains the NLRC4 (NLR family, CARD domain containing 4) protein was previously shown to be activated in response to two distinct bacterial proteins, flagellin and PrgJ, a conserved component of pathogen-associated type III secretion systems. However, direct binding between NLRC4 and flagellin or PrgJ has never been demonstrated. A homologue of NLRC4, NAIP5 (NLR family, apoptosis inhibitory protein 5), has been implicated in activation of NLRC4 (refs 7-11), but is widely assumed to have only an auxiliary role, as NAIP5 is often dispensable for NLRC4 activation. However, Naip5 is a member of a small multigene family, raising the possibility of redundancy and functional specialization among Naip genes. Here we show in mice that different NAIP paralogues determine the specificity of the NLRC4 inflammasome for distinct bacterial ligands. In particular, we found that activation of endogenous NLRC4 by bacterial PrgJ requires NAIP2, a previously uncharacterized member of the NAIP gene family, whereas NAIP5 and NAIP6 activate NLRC4 specifically in response to bacterial flagellin. We dissected the biochemical mechanism underlying the requirement for NAIP proteins by use of a reconstituted NLRC4 inflammasome system. We found that NAIP proteins control ligand-dependent oligomerization of NLRC4 and that the NAIP2-NLRC4 complex physically associates with PrgJ but not flagellin, whereas NAIP5-NLRC4 associates with flagellin but not PrgJ. Our results identify NAIPs as immune sensor proteins and provide biochemical evidence for a simple receptor-ligand model for activation of the NAIP-NLRC4 inflammasomes.
© 2011 Macmillan Publishers Limited. All rights reserved
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Figure 1. NAIP2 is required in macrophages for inflammasome activation in response to PrgJ
(a–c) Primary bone-marrow derived macrophages expressing shRNAs targeting NAIP2 (or controls) were infected with flagellin-deficient Listeria monocytogenes (MOI = 5) expressing a secreted ActA100-PrgJ (pPrgJ) or ActA100-FlaA (pFlaA) fusion protein under IPTG-inducible control. (a, b) Cell death (± s.d.) was measured in triplicate by LDH release 6 hours after infection, or (c) Active CASP1 (p10) was measured by western blotting of cell supernatants. (d, e) NAIP2 knockdown cells were infected with wildtype or flagellin-deficient (FliC/FljB−) Salmonella Typhimurium and inflammasome activation was measured by (d) LDH release (± s.d.) at 3h after infection or (e) CASP1 processing. Data shown are representative of two (c, e) or three (a, b, d) independent experiments. *, p<0.02 as compared to scramble (Student’s t-test, two-tailed).
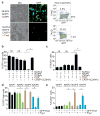
Figure 2. Reconstitution of the NAIP5/NLRC4 inflammasome in 293T cells
(a) GFP-marked expression vectors encoding NLRC4, NAIP5, CASP1 and/or flagellin (FlaA) were transiently transfected into 293T cells. Cells were imaged for differential interference contrast (DIC) and GFP fluorescence 48 hours later. Dead cells were stained with 7AAD. (b) GFP-high cells and (c) 7AAD positive cells were quantified (± s.d.) as in a, but with specific expression vectors omitted from the transfection as indicated. CASP1(C284A) is a catalytically dead mutant. (d, e) 293T cells were transfected as indicated and analyzed as above. Data shown (± s.d.) are representative of at least three independent experiments. *, p<0.02 (Student’s t test, two tailed); ns, not significant.
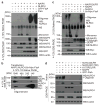
Figure 3. NAIP5 is required for formation of a hetero-oligomeric complex that contains NLRC4, NAIP5 and flagellin
(a) 293T cells were transfected as indicated, followed by analysis by Blue Native-PAGE or SDS-PAGE, and western blotting. *NS, non-specific band. (b) 293T cells were transfected as indicated and lysates were separated by a first dimension of Blue Native-PAGE followed by a second dimension of SDS-PAGE. (c, d) 293T cells were transfected as indicated and samples were processed and analyzed as in a. Data shown are representative of at least three independent experiments.
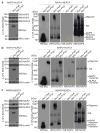
Figure 4. NAIP Paralogs Confer Specificity to the NLRC4 Inflammasome
(a) 293T cells were co-transfected with wild-type NAIP5 and NLRC4, alone or in combination with 6x-Myc-FlaA or 6x-Myc-PrgJ followed by Blue Native PAGE 48 hours later. *NS, non-specific band. Whole cell lysates were also separated by conventional 4–12% SDS-PAGE to control for expression of each transfected gene construct (left panel). (b) 293T cells were transfected with wild-type NAIP2 and NLRC4 and analyzed as in a. (c) 293T cells were transfected with wild-type NAIP6 and NLRC4, and analyzed as in a. Data shown are representative of at least three independent experiments.
Comment in
- Inflammasome: NAIPs: pathogen-sensing proteins.
Leavy O. Leavy O. Nat Rev Immunol. 2011 Sep 9;11(10):644. doi: 10.1038/nri3069. Nat Rev Immunol. 2011. PMID: 21904388 No abstract available. - Immunology: recognition of a unique partner.
Monack DM. Monack DM. Nature. 2011 Sep 28;477(7366):543-4. doi: 10.1038/477543a. Nature. 2011. PMID: 21956324 No abstract available.
Similar articles
- The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus.
Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, Shao F. Zhao Y, et al. Nature. 2011 Sep 14;477(7366):596-600. doi: 10.1038/nature10510. Nature. 2011. PMID: 21918512 - Differential requirements for NAIP5 in activation of the NLRC4 inflammasome.
Lightfield KL, Persson J, Trinidad NJ, Brubaker SW, Kofoed EM, Sauer JD, Dunipace EA, Warren SE, Miao EA, Vance RE. Lightfield KL, et al. Infect Immun. 2011 Apr;79(4):1606-14. doi: 10.1128/IAI.01187-10. Epub 2011 Jan 31. Infect Immun. 2011. PMID: 21282416 Free PMC article. - Broad detection of bacterial type III secretion system and flagellin proteins by the human NAIP/NLRC4 inflammasome.
Reyes Ruiz VM, Ramirez J, Naseer N, Palacio NM, Siddarthan IJ, Yan BM, Boyer MA, Pensinger DA, Sauer JD, Shin S. Reyes Ruiz VM, et al. Proc Natl Acad Sci U S A. 2017 Dec 12;114(50):13242-13247. doi: 10.1073/pnas.1710433114. Epub 2017 Nov 27. Proc Natl Acad Sci U S A. 2017. PMID: 29180436 Free PMC article. - Sensing bacterial infections by NAIP receptors in NLRC4 inflammasome activation.
Gong YN, Shao F. Gong YN, et al. Protein Cell. 2012 Feb;3(2):98-105. doi: 10.1007/s13238-012-2028-3. Epub 2012 Mar 17. Protein Cell. 2012. PMID: 22426978 Free PMC article. Review.
Cited by
- Mechanism and Application Prospects of NLRC3 Regulating cGAS-STING Pathway in Lung Cancer Immunotherapy.
Wang Q, Ren Z, Zhao J, Zheng T, Tong L, Liu J, Dai Z, Tang S. Wang Q, et al. Int J Med Sci. 2024 Oct 7;21(13):2613-2622. doi: 10.7150/ijms.102328. eCollection 2024. Int J Med Sci. 2024. PMID: 39439455 Free PMC article. Review. - Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila.
Case CL, Kohler LJ, Lima JB, Strowig T, de Zoete MR, Flavell RA, Zamboni DS, Roy CR. Case CL, et al. Proc Natl Acad Sci U S A. 2013 Jan 29;110(5):1851-6. doi: 10.1073/pnas.1211521110. Epub 2013 Jan 10. Proc Natl Acad Sci U S A. 2013. PMID: 23307811 Free PMC article. - Pseudomonas aeruginosa pili and flagella mediate distinct binding and signaling events at the apical and basolateral surface of airway epithelium.
Bucior I, Pielage JF, Engel JN. Bucior I, et al. PLoS Pathog. 2012;8(4):e1002616. doi: 10.1371/journal.ppat.1002616. Epub 2012 Apr 5. PLoS Pathog. 2012. PMID: 22496644 Free PMC article. - Innate immune recognition of flagellin limits systemic persistence of Brucella.
Terwagne M, Ferooz J, Rolán HG, Sun YH, Atluri V, Xavier MN, Franchi L, Núñez G, Legrand T, Flavell RA, De Bolle X, Letesson JJ, Tsolis RM. Terwagne M, et al. Cell Microbiol. 2013 Jun;15(6):942-960. doi: 10.1111/cmi.12088. Epub 2013 Jan 7. Cell Microbiol. 2013. PMID: 23227931 Free PMC article. - Inflammasome Complexes: Emerging Mechanisms and Effector Functions.
Rathinam VA, Fitzgerald KA. Rathinam VA, et al. Cell. 2016 May 5;165(4):792-800. doi: 10.1016/j.cell.2016.03.046. Cell. 2016. PMID: 27153493 Free PMC article. Review.
References
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. - PubMed
- Ting JP, Kastner DL, Hoffman HM. CATERPILLERs, pyrin and hereditary immunological disorders. Nat Rev Immunol. 2006;6:183–195. - PubMed
- Franchi L, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. - PubMed
- Miao EA, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI075039-04/AI/NIAID NIH HHS/United States
- R37 AI075039/AI/NIAID NIH HHS/United States
- AI075039/AI/NIAID NIH HHS/United States
- R01 AI080749-03/AI/NIAID NIH HHS/United States
- AI063302/AI/NIAID NIH HHS/United States
- P01 AI063302/AI/NIAID NIH HHS/United States
- R01 AI080749/AI/NIAID NIH HHS/United States
- AI080749/AI/NIAID NIH HHS/United States
- R01 AI075039/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials