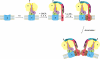Mitochondrial ATP synthase: architecture, function and pathology - PubMed (original) (raw)
Review
Mitochondrial ATP synthase: architecture, function and pathology
An I Jonckheere et al. J Inherit Metab Dis. 2012 Mar.
Abstract
Human mitochondrial (mt) ATP synthase, or complex V consists of two functional domains: F(1), situated in the mitochondrial matrix, and F(o), located in the inner mitochondrial membrane. Complex V uses the energy created by the proton electrochemical gradient to phosphorylate ADP to ATP. This review covers the architecture, function and assembly of complex V. The role of complex V di-and oligomerization and its relation with mitochondrial morphology is discussed. Finally, pathology related to complex V deficiency and current therapeutic strategies are highlighted. Despite the huge progress in this research field over the past decades, questions remain to be answered regarding the structure of subunits, the function of the rotary nanomotor at a molecular level, and the human complex V assembly process. The elucidation of more nuclear genetic defects will guide physio(patho)logical studies, paving the way for future therapeutic interventions.
Figures
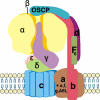
Fig. 1
Human mitochondrial ATP synthase, or complex V, consists of two functional domains, F1 and Fo. F1 comprises 5 different subunits (three α, three β, and one γ, δ and ε) and is situated in the mitochondrial matrix. Fo contains subunits c, a, b, d, F6, OSCP and the accessory subunits e, f, g and A6L. F1 subunits γ, δ and ε constitute the central stalk of complex V. Subunits b, d, F6 and OSCP form the peripheral stalk. Protons pass from the intermembrane space to the matrix through Fo, which transfers the energy created by the proton electrochemical gradient to F1, where ADP is phosphorylated to ATP. One β subunit is taken out to visualize the central stalk
Fig. 2
Complex V assembly and dimerization. The current working model is based on assembly of the c-ring followed by binding of F1, the stator arm, and finally of subunits a and A6L. Two ATP synthase monomers dimerize via the Fo sector, where subunits a, e, g, b and A6L stabilize the monomer-monomer interface
Similar articles
- The spatio-temporal organization of mitochondrial F1FO ATP synthase in cristae depends on its activity mode.
Salewskij K, Rieger B, Hager F, Arroum T, Duwe P, Villalta J, Colgiati S, Richter CP, Psathaki OE, Enriquez JA, Dellmann T, Busch KB. Salewskij K, et al. Biochim Biophys Acta Bioenerg. 2020 Jan 1;1861(1):148091. doi: 10.1016/j.bbabio.2019.148091. Epub 2019 Nov 27. Biochim Biophys Acta Bioenerg. 2020. PMID: 31669489 - Stepwise assembly of dimeric F(1)F(o)-ATP synthase in mitochondria involves the small F(o)-subunits k and i.
Wagner K, Perschil I, Fichter CD, van der Laan M. Wagner K, et al. Mol Biol Cell. 2010 May 1;21(9):1494-504. doi: 10.1091/mbc.e09-12-1023. Epub 2010 Mar 10. Mol Biol Cell. 2010. PMID: 20219971 Free PMC article. - Atypical subunit composition of the chlorophycean mitochondrial F1FO-ATP synthase and role of Asa7 protein in stability and oligomycin resistance of the enzyme.
Lapaille M, Escobar-Ramírez A, Degand H, Baurain D, Rodríguez-Salinas E, Coosemans N, Boutry M, Gonzalez-Halphen D, Remacle C, Cardol P. Lapaille M, et al. Mol Biol Evol. 2010 Jul;27(7):1630-44. doi: 10.1093/molbev/msq049. Epub 2010 Feb 15. Mol Biol Evol. 2010. PMID: 20156838 - Structures and interactions of proteins involved in the coupling function of the protonmotive F(o)F(1)-ATP synthase.
Gaballo A, Zanotti F, Papa S. Gaballo A, et al. Curr Protein Pept Sci. 2002 Aug;3(4):451-60. doi: 10.2174/1389203023380558. Curr Protein Pept Sci. 2002. PMID: 12370007 Review. - IF(1): setting the pace of the F(1)F(o)-ATP synthase.
Campanella M, Parker N, Tan CH, Hall AM, Duchen MR. Campanella M, et al. Trends Biochem Sci. 2009 Jul;34(7):343-50. doi: 10.1016/j.tibs.2009.03.006. Epub 2009 Jun 24. Trends Biochem Sci. 2009. PMID: 19559621 Review.
Cited by
- One-step automated bioprinting-based method for cumulus-oocyte complex microencapsulation for 3D in vitro maturation.
Mastrorocco A, Cacopardo L, Martino NA, Fanelli D, Camillo F, Ciani E, Roelen BAJ, Ahluwalia A, Dell'Aquila ME. Mastrorocco A, et al. PLoS One. 2020 Sep 11;15(9):e0238812. doi: 10.1371/journal.pone.0238812. eCollection 2020. PLoS One. 2020. PMID: 32915922 Free PMC article. - Opposite rotation directions in the synthesis and hydrolysis of ATP by the ATP synthase: hints from a subunit asymmetry.
Nesci S, Trombetti F, Ventrella V, Pagliarani A. Nesci S, et al. J Membr Biol. 2015 Apr;248(2):163-9. doi: 10.1007/s00232-014-9760-y. Epub 2015 Feb 6. J Membr Biol. 2015. PMID: 25655107 Review. - Mitochondrial Stress in Metabolic Inflammation: Modest Benefits and Full Losses.
Yuan Q, Zeng ZL, Yang S, Li A, Zu X, Liu J. Yuan Q, et al. Oxid Med Cell Longev. 2022 Nov 22;2022:8803404. doi: 10.1155/2022/8803404. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36457729 Free PMC article. Review. - Power(2): the power of yeast genetics applied to the powerhouse of the cell.
Rutter J, Hughes AL. Rutter J, et al. Trends Endocrinol Metab. 2015 Feb;26(2):59-68. doi: 10.1016/j.tem.2014.12.002. Epub 2015 Jan 12. Trends Endocrinol Metab. 2015. PMID: 25591985 Free PMC article. Review. - Deletion of the ATP2 Gene in Candida albicans Blocks Its Escape From Macrophage Clearance.
Zhang Y, Tang C, Zhang Z, Li S, Zhao Y, Weng L, Zhang H. Zhang Y, et al. Front Cell Infect Microbiol. 2021 Apr 16;11:643121. doi: 10.3389/fcimb.2021.643121. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33937095 Free PMC article.
References
- Abu-Amero KK, Bosley TM. Mitochondrial abnormalities in patients with LHON-like optic neuropathies. Invest Ophthalmol Vis Sci. 2006;47:4211–4220. - PubMed
- Ackerman SH. Atp11p and Atp12p are chaperones for F(1)-ATPase biogenesis in mitochondria. Biochim Biophys Acta. 2002;1555:101–105. - PubMed
- Adachi K, Oiwa K, Nishizaka T, et al. Coupling of rotation and catalysis in F(1)-ATPase revealed by single-molecule imaging and manipulation. Cell. 2007;130:309–321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous