Sustained suppression by Foxp3+ regulatory T cells is vital for infectious transplantation tolerance - PubMed (original) (raw)
Sustained suppression by Foxp3+ regulatory T cells is vital for infectious transplantation tolerance
Adrian R Kendal et al. J Exp Med. 2011.
Abstract
A paradigm shift in immunology has been the recent discovery of regulatory T cells (T reg cells), of which CD4(+)Foxp3(+) cells are proven as essential to self-tolerance. Using transgenic B6.Foxp3(hCD2) mice to isolate and ablate Foxp3(+) T reg cells with an anti-hCD2 antibody, we show for the first time that CD4(+)Foxp3(+) cells are crucial for infectious tolerance induced by nonablative anti-T cell antibodies. In tolerant animals, Foxp3(+) T reg cells are constantly required to suppress effector T cells still capable of causing tissue damage. Tolerated tissue contains T cells that are capable of rejecting it, but are prevented from doing so by therapeutically induced Foxp3(+) T reg cells. Finally, Foxp3(+) cells have been confirmed as the critical missing link through which infectious tolerance operates in vivo. Peripherally induced Foxp3(+) cells sustain tolerance by converting naive T cells into the next generation of Foxp3(+) cells. Empowering Foxp3(+) regulatory T cells in vivo offers a tractable route to avoid and correct tissue immunopathology.
Figures
Figure 1.
Ablation of FoxP3+ T cells with an anti–human CD2 antibody. (A) Ablation of Foxp3+ cells after B6.Foxp3hCD2 mice received 1 mg YTH655 (at anti-hCD2 IgG2b mAb) i.p. in indicated organs. Three mice/group/time point are shown. (B) Foxp3+ cells after seven daily i.p. injections of 250 µg YTH655. P < 0.001. Three mice/group/time point are shown. (C) Flow cytometry analysis of spleen, lymph, and blood cells 4 wk after 2–10 million B6.Foxp3hCD2 splenocytes were injected i.v. into lymphocyte-deficient B6.RAG−/− recipients with 1 mg anti-hCD2 (squares; n = 16 composite of four experiments). **, P < 0.01 for the ablative effect of antibody compared to controls. (D) Splenocytes from B6.Foxp3hCD2 mice pretreated (open triangles, n = 6) or not (open circles, n = 3) with 7 × 250 µg anti-hCD2 mAb (30–40% of Foxp3+ T cells survived) were harvested and transferred into B6 RAG−/− mice with 1 mg anti-hCD2. P < 0.0001 compared with control (circles, n = 3). In all cases, horizontal bars represent the arithmetic mean and error bars represent SEM.
Figure 2.
Loss of transplant tolerance and suppression on adoptive transfer after ablation of FoxP3+ T cells. (A) (CBAxB6hCD2)F1 mice can be made tolerant to multiple minor mismatched Balb/K skin grafts with anti-CD4/CD8/CD40L blocking mAb (squares, n = 23; P < 0.0001 compared with control; circles, _n_ = 4). The skin grafts were accepted for >150 d and were not rejected despite challenge grafting at day 60 (arrow). (B) Tolerant spleen cells were harvested from another cohort of (CBAxB6hCD2)F1 mice that had accepted a Balb/K graft for at least 60 d and transferred into RAG−/− recipients in conjunction with a challenge Balb/K graft. 20 million tolerant splenocytes from male (CBAxB6hCD2)F1 mice suppressed the addition of five million naive splenocytes (triangles, n = 6) and one million primed splenocytes (circles, n = 5). This suppression was dependent on Foxp3+ T cells because anti-hCD2 mAb led to rapid rejection of the skin grafts. P = 0.0026 (n = 9) and P = 0.0168 (n = 5), respectively (one mouse in each group was euthanized). (C) 20 million tolerant splenocytes from male (CBAxB6hCD2)F1 mice that accepted the first Balb/K graft also accepted a Balb/K skin graft when adoptively transferred into lymphocyte deplete (RAG−/−) recipients. When 1 mg anti-hCD2 was co-administered, the grafts were rejected. P = 0.0008 (n = 11). (D) 20 million tolerant splenocytes from female (CBAxB6hCD2)F1 mice that accepted the first Balb/K graft also accepted a Balb/K skin graft when adoptively transferred into lymphocyte deplete (RAG−/−) recipients with (open triangles, n = 8) or without (closed triangles, n = 3) anti-hCD2 mAb. In the same experiment, 20 million tolerant splenocytes from male (CBAxB6hCD2)F1 mice rejected the second Balb/K graft when co-administered with anti-hCD2 mAb (circles, n = 4; P = 0.0047 one mouse was euthanized).
Figure 3.
Loss of transplant tolerance after ablation of Foxp3+ T cells in tolerant hosts. (A) Male (CBAxB6hCD2)F1 mice treated with anti-CD4/CD8/CD40L blocking mAb (3 × 3 mg doses over 7 d) accepted an MHC mismatched Balb/C skin (n = 27; P < 0.0001). (B) After 100 d, a challenge Balb/C skin graft was transplanted. Mice that received ablative anti-hCD2 mAb to deplete Foxp3+ T cells (squares, n = 16) rapidly rejected the challenge graft. MST 12.0 versus 47.0 d of control group (circles, n = 11), P < 0.0001. (C) The original tolerizing Balb/C graft was also rapidly rejected when mice received anti-hCD2 mAb (squares, n = 16). MST (+100d) 17.5 versus 38.0 d of control group (circles, n = 11), P = 0.0012. (D) Even without a challenge graft, mice that received anti-hCD2 mAb to ablate Foxp3+ T cells (downward triangles, n = 4; squares, n = 9) rejected a Balb/C graft that had been well tolerated for 50 d, with (P = 0.045) or without (P < 0.0001) a co-transplanted syngeneic graft.
Figure 4.
Foxp3+ iTreg cells are induced in vivo by co-receptor blockade, and FoxP3+ iTreg cells induced by TGF in vitro are suppressive in vivo. (A) Female transgenic RAG−/− Marilyn.Foxp3hCD2 mice can be tolerized to male B6 RAG−/− skin by anti-CD4 blocking mAb (squares, n = 10; P = 0.0001). (B) De novo hCD2+Foxp3+ T reg cells were found in the spleen, draining, and mesenteric lymph nodes, but not the thymus, of Marilyn.Foxp3hCD2 mice that accepted a male graft for at least 60 d but not in untreated rejecting RAG−/− Marilyn.Foxp3hCD2 mice (P < 0.0001). (C) 5 × 105 TGF-β–conditioned male H-Y antigen-specific hCD2+ CD4+ RAG−/− Marilyn.Foxp3hCD2 (DBYT) cells failed to reject a male CBA.Ca RAG−/− graft (circles, n = 7) when injected into B6 RAG−/− recipients compared with 5 × 105 hCD2− DBYT cells (open squares, n = 11; P = 0.0003) or 1 × 105 naive CD4+ RAG−/− Marilyn.Foxp3hCD2 cells (open triangles, n = 6; P = 0.0006). The hCD2− DBYT group consists of mice injected with MoFlow-sorted hCD2− DBYT cells. Alone, n = 3; plus anti-hCD2 mAb, n = 4; plus isotype control mAb, n = 4. (D) 5 × 105 unsorted (DBYT) cells (squares, n = 4) and 5 × 105 hCD2+ cells (diamond, n = 5) fail to reject a male CBA.Ca RAG−/− graft when injected into B6 RAG−/− recipients. Elimination of hCD2+Foxp3+ T cells, either by anti-hCD2 mAb (open squares, n = 5; P = 0.0095) or MoFlow cell sorting of hCD2− cells (open triangles, n = 5; P = 0.0039), results in graft rejection. A challenge graft co-transplanted with 1 × 105 naive RAG−/− MarilynWT CD4+ cells after 30 d (arrow) fails to break tolerance and is itself accepted long term. (E) A positive control group is included to demonstrate that 1 × 105 naive RAG−/− MarilynWT CD4+ cells normally reject male skin when injected into naive RAG−/− mice (open circles, n = 3; P = 0.0062). **, P < 0.01; ***, P < 0.001.
Figure 5.
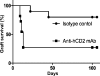
FoxP3+ T cells within grafted tissue contributed to the survival of tolerated skin grafts transferred onto lymphopenic recipients. Male B6 RAG−/− grafts that were tolerated by anti-CD4 mAb–treated female RAG−/− Marilyn.Foxp3hCD2 mice (Fig. 4 A) were then retransplanted onto lymphocyte-deficient female B6 RAG−/− recipients. Ablation with anti-hCD2 mAb (squares, n = 11) resulted in rapid graft rejection compared with isotype control mAb (circles, n = 10; P = 0.0040).
Figure 6.
In vitro–generated Foxp3+ iTreg cells prevented graft rejection by naive CD4 T cells and allowed some naive T cells to become Foxp3+ themselves. Female B6.RAG−/− received a male CBA.RAG−/− skin graft in conjunction with either 5 × 105 in vitro–cultured female hCD2+ RAG−/− Marilyn.Foxp3hCD2 DBYT cells (squares, n = 6), 1 × 105 naive CD4+ cells from RAG−/− MarilynWT mice (circles, n = 6), or a mixture of both (diamonds, n = 8). (A) All of the mice that received 1 × 105 naive CD4+ cells rejected the male skin compared with none of those that received 5 × 105 hCD2+ cells (P = 0.0066) and half that received a mixture (P = 0.0247). After 100 d, the mice were killed and Foxp3+ cell composition was analyzed. (B) Total white cell spleen counts were similar between the three groups, but there were more CD4+ cells in mice that received a mixture of hCD2+ and naive CD4+ cells. (C) FACS analysis demonstrated conversion of naive CD4+ cells into Foxp3+ T cells. Over 98% of Foxp3+ cells in mice that received 5 × 105 isolated hCD2+ RAG−/− Marilyn.Foxp3hCD2 cells alone were hCD2+, whereas 18% of Foxp3+ cells in the draining lymph and 24% in the spleen of mice that received a 5:1 mixture (and had accepted the male graft) were hCD2 negative (P = 0.0009). Error bars represent SEM.
Similar articles
- Oral tolerance induction with antigen conjugated to cholera toxin B subunit generates both Foxp3+CD25+ and Foxp3-CD25- CD4+ regulatory T cells.
Sun JB, Raghavan S, Sjöling A, Lundin S, Holmgren J. Sun JB, et al. J Immunol. 2006 Dec 1;177(11):7634-44. doi: 10.4049/jimmunol.177.11.7634. J Immunol. 2006. PMID: 17114433 - Pancreatic islets induce CD4(+) [corrected] CD25(-)Foxp3(+) [corrected] T-cell regulated tolerance to HY-mismatched skin grafts.
Yoon IH, Choi SE, Kim YH, Yang SH, Park JH, Park CS, Kim Y, Kim JS, Kim SJ, Simpson E, Park CG. Yoon IH, et al. Transplantation. 2008 Nov 27;86(10):1352-60. doi: 10.1097/TP.0b013e31818aa43c. Transplantation. 2008. PMID: 19034003 - Transplantation survival is maintained by granzyme B+ regulatory cells and adaptive regulatory T cells.
Gondek DC, Devries V, Nowak EC, Lu LF, Bennett KA, Scott ZA, Noelle RJ. Gondek DC, et al. J Immunol. 2008 Oct 1;181(7):4752-60. doi: 10.4049/jimmunol.181.7.4752. J Immunol. 2008. PMID: 18802078 Free PMC article. - Plasticity of T(reg) cells: is reprogramming of T(reg) cells possible in the presence of FOXP3?
Beyer M, Schultze JL. Beyer M, et al. Int Immunopharmacol. 2011 May;11(5):555-60. doi: 10.1016/j.intimp.2010.11.024. Epub 2010 Nov 27. Int Immunopharmacol. 2011. PMID: 21115121 Review. - The TGF-beta1/Foxp3 regulatory axis in immune self-tolerance: implications for health and disease.
Pyzik M, Piccirillo CA. Pyzik M, et al. Inflamm Allergy Drug Targets. 2006 Sep;5(3):167-77. doi: 10.2174/187152806778256089. Inflamm Allergy Drug Targets. 2006. PMID: 16918480 Review.
Cited by
- The environment of regulatory T cell biology: cytokines, metabolites, and the microbiome.
Hoeppli RE, Wu D, Cook L, Levings MK. Hoeppli RE, et al. Front Immunol. 2015 Feb 18;6:61. doi: 10.3389/fimmu.2015.00061. eCollection 2015. Front Immunol. 2015. PMID: 25741338 Free PMC article. Review. - 4-1BB Signaling in Conventional T Cells Drives IL-2 Production That Overcomes CD4+CD25+FoxP3+ T Regulatory Cell Suppression.
Barsoumian HB, Yolcu ES, Shirwan H. Barsoumian HB, et al. PLoS One. 2016 Apr 6;11(4):e0153088. doi: 10.1371/journal.pone.0153088. eCollection 2016. PLoS One. 2016. PMID: 27049955 Free PMC article. - Short Report: CAR Tregs mediate linked suppression and infectious tolerance in islet transplantation.
Wardell CM, Fung VCW, Chen E, Haque M, Gillies J, Spanier JA, Mojibian M, Fife BT, Levings MK. Wardell CM, et al. bioRxiv [Preprint]. 2024 Apr 10:2024.04.06.588414. doi: 10.1101/2024.04.06.588414. bioRxiv. 2024. PMID: 38645184 Free PMC article. Preprint. - Differential sensitivity of regulatory and effector T cells to cell death: a prerequisite for transplant tolerance.
You S. You S. Front Immunol. 2015 May 19;6:242. doi: 10.3389/fimmu.2015.00242. eCollection 2015. Front Immunol. 2015. PMID: 26042125 Free PMC article. Review. - Long-term remission of diabetes in NOD mice is induced by nondepleting anti-CD4 and anti-CD8 antibodies.
Yi Z, Diz R, Martin AJ, Morillon YM, Kline DE, Li L, Wang B, Tisch R. Yi Z, et al. Diabetes. 2012 Nov;61(11):2871-80. doi: 10.2337/db12-0098. Epub 2012 Jun 29. Diabetes. 2012. PMID: 22751694 Free PMC article.
References
- Battaglia M., Stabilini A., Migliavacca B., Horejs-Hoeck J., Kaupper T., Roncarolo M.G. 2006. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J. Immunol. 177:8338–8347 - PubMed
- Benghiat F.S., Graca L., Braun M.Y., Detienne S., Moore F., Buonocore S., Flamand V., Waldmann H., Goldman M., Le Moine A. 2005. Critical influence of natural regulatory CD25+ T cells on the fate of allografts in the absence of immunosuppression. Transplantation. 79:648–654 10.1097/01.TP.0000155179.61445.78 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials