Large collective motions regulate the functional properties of glutamate transporter trimers - PubMed (original) (raw)
Large collective motions regulate the functional properties of glutamate transporter trimers
Jie Jiang et al. Proc Natl Acad Sci U S A. 2011.
Abstract
Glutamate transporters clear synaptically released glutamate to maintain precise communication between neurons and limit glutamate neurotoxicity. Although much progress has been made on the topology, structure, and function of these carriers, few studies have addressed large-scale structural motions collectively associated with substrate transport. Here we show that a series of single cysteine substitutions in the helical hairpin HP2 of excitatory amino acid transporter 1 form intersubunit disulfide cross-links within the trimer. After cross-linking, substrate uptake, but not substrate-activated anion conductance, is completely inhibited in these mutants. These disulfide bridges link residue pairs > 40 Å apart in the outward-facing crystal structure, and can be explained by concerted subunit movements predicted by the anisotropic network model (ANM). The existence of these global motions is further supported by the observation that single cysteine substitutions at the extracellular part of the transmembrane domain 8 can also be cross-linked by copper phenanthroline as predicted by the ANM. Interestingly, the transport domain in the un-cross-linked subunit of the trimer assumes an inward-facing orientation, suggesting that individual subunits potentially undergo separate transitions between outward- and inward-facing forms, rather than an all-or-none transition of the three subunits, a mechanism also supported by ANM-predicted intrinsic dynamics. These results shed light on how large collective motions contribute to the functional dynamics of glutamate transporters.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
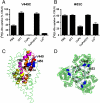
Inhibition of substrate transport by CuPh and cadmium in mutants V449C and I453C. COS7 cells were treated with 20 mM DTT, 300 μM CuPh, or CuPh followed by DTT, for 5 min. For CuPh preparation, CuSO4 and 1,10-phenanthroline were mixed in a 1∶2 ratio prior to use. Uptake assays were performed for 10 min at room temperature using 5 μM L-[3H] glutamate as substrate. The effect of cadmium on transport activity was determined by including 100 μM cadmium chloride in the uptake solution. Data for V449C (A) and I453C (B) are expressed as the percent of uptake activity relative to the CSLS control. (C) Locations of V449 and I453 on the helix H2b of the HP2 hairpin, shown for one subunit (side view). (D) Estimated distances between the three V449 residues, and the three I453 residues in an EAAT1 trimer (top view). The illustrations are based on the GltPh crystal structure in the outward-facing state (PDB ID 1XFH) and are made using the software Pymol.
Fig. 2.
Single cysteine substitutions at the N terminus of the helix HP2b are more reactive and form spontaneous cross-links. (A) The effects of 300 μM CuPh on the
l
-glutamate transport activity of a series of cysteine substitution mutants. (B) Substrate transport by mutant transporters after addition of 20 mM DTT for 5 min. Uptake assays were performed as described in Fig. 1 and data are represented as the percentage of untreated controls in two to four experiments done in triplicate.
Fig. 3.
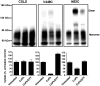
CuPh-induced cross-linking occurs between two subunits. Transporters were expressed in Xenopus oocytes and treated as indicated. Oocyte membranes were lysed and separated by nonreducing gradient SDS-PAGE and subjected to Western blotting analyses. Uptake assays in oocytes were carried out at room temperature using 5 μM L-[3H] glutamate as substrate for 15 min, and data are normalized to untreated controls.
Fig. 4.
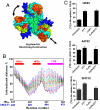
Large collective motions of glutamate transporters predicted by ANM analysis. (A) Schematic representation of the asymmetric stretching/contraction mode viewed from the EC face. The arrows indicate the direction of motion of the three subunits. (B) Intersubunit distances of residues in HP2 and TM8 (based on GltPh C_α_ atoms between two approaching subunits) are significantly altered along the asymmetric stretching/contraction mode. The thick red curve refers to the equilibrium distances derived from the X-ray structure (PDB ID 1XFH), and the series of curves above and below refer to different extents of deformation along this softest mode in the positive and negative directions. EAAT1 residue numbers are denoted in blue, below the abscissa. (C) Inhibition of substrate transport in mutants with single cysteines substituted into the N terminus of TM8. Cross-linking and uptake assays were performed as described in Fig. 1.
Fig. 5.
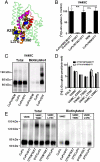
The un-cross-linked subunit is in inward-facing conformation after V449C cross-linking. (A) Location of residues (V449, A355, T368, L376, and V390) in the context of the inward-facing GltPh crystal structure (PDB ID 3KBC). (B and D) COS7 cells were treated for 5 min with 300 μM CuPh, 1 mM MTSET, 0.5 mM NEM, or 10 mM DTT as indicated. Uptake assays were performed as described in Fig. 1. After treatments, surface proteins were biotinylated with maleimide-PEO2-biotin (C) or maleimide-biotin (E) and subjected to Western blotting analyses. n.s., not significant; *, p < 0.05.
Fig. 6.
Cumulative overlap of ANM modes for transitions between pairs of conformations. (A) Ribbon diagrams of the four structures used for transport domain transitions. The all-inward (0/3) and all-outward conformations (3/0) represent the 3KBC and 1XFH structures, respectively. In the intermediate structure model designated as 2/1, one subunit faces inward (cyan), whereas in 1/2, two subunits face inward (green and cyan). (B) A schematic representation of the passages between different conformations. (C and D) Cumulative overlap of the ANM modes with the deformation vector between pairs of conformations indicated by the labels on the paths. The control curves refer to the case of randomly oriented modes that contribute equally to conformational transitions.
Fig. 7.
V449C retains anion conductance after cross-linking. Using a two-electrode voltage clamp, currents from oocytes expressing CSLS or V449C were recorded in normal chloride-containing solution (A) and in a solution in which chloride was replaced by  (B). Recordings were made before (▪) and after (▴) application of 300 μM CuPh (5 min), as well as after initial treatment with 20 mM DTT (5 min, ○). The currents obtained in the absence of glutamate were subtracted from those elicited by 1 mM
(B). Recordings were made before (▪) and after (▴) application of 300 μM CuPh (5 min), as well as after initial treatment with 20 mM DTT (5 min, ○). The currents obtained in the absence of glutamate were subtracted from those elicited by 1 mM
l
-glutamate. Currents were normalized to the control currents obtained at -100 mV.
Similar articles
- Inward-facing conformation of glutamate transporters as revealed by their inverted-topology structural repeats.
Crisman TJ, Qu S, Kanner BI, Forrest LR. Crisman TJ, et al. Proc Natl Acad Sci U S A. 2009 Dec 8;106(49):20752-7. doi: 10.1073/pnas.0908570106. Epub 2009 Nov 19. Proc Natl Acad Sci U S A. 2009. PMID: 19926849 Free PMC article. - Substrate-Induced Motion between TM4 and TM7 of the Glutamate Transporter EAAT1 Revealed by Paired Cysteine Mutagenesis.
Zhang W, Zhang X, Qu S. Zhang W, et al. Mol Pharmacol. 2019 Jan;95(1):33-42. doi: 10.1124/mol.118.113183. Epub 2018 Oct 22. Mol Pharmacol. 2019. PMID: 30348896 - Structural rearrangements at the translocation pore of the human glutamate transporter, EAAT1.
Leighton BH, Seal RP, Watts SD, Skyba MO, Amara SG. Leighton BH, et al. J Biol Chem. 2006 Oct 6;281(40):29788-96. doi: 10.1074/jbc.M604991200. Epub 2006 Jul 28. J Biol Chem. 2006. PMID: 16877378 - The dual-function glutamate transporters: structure and molecular characterisation of the substrate-binding sites.
Kanner BI, Borre L. Kanner BI, et al. Biochim Biophys Acta. 2002 Sep 10;1555(1-3):92-5. doi: 10.1016/s0005-2728(02)00260-8. Biochim Biophys Acta. 2002. PMID: 12206897 Review. - Slips, leaks and channels in glutamate transporters.
Vandenberg RJ, Huang S, Ryan RM. Vandenberg RJ, et al. Channels (Austin). 2008 Jan-Feb;2(1):51-8. doi: 10.4161/chan.2.1.6047. Epub 2008 Apr 2. Channels (Austin). 2008. PMID: 18690049 Review.
Cited by
- Shared dynamics of LeuT superfamily members and allosteric differentiation by structural irregularities and multimerization.
Ponzoni L, Zhang S, Cheng MH, Bahar I. Ponzoni L, et al. Philos Trans R Soc Lond B Biol Sci. 2018 Jun 19;373(1749):20170177. doi: 10.1098/rstb.2017.0177. Philos Trans R Soc Lond B Biol Sci. 2018. PMID: 29735731 Free PMC article. - Protein Ensembles: How Does Nature Harness Thermodynamic Fluctuations for Life? The Diverse Functional Roles of Conformational Ensembles in the Cell.
Wei G, Xi W, Nussinov R, Ma B. Wei G, et al. Chem Rev. 2016 Jun 8;116(11):6516-51. doi: 10.1021/acs.chemrev.5b00562. Epub 2016 Jan 25. Chem Rev. 2016. PMID: 26807783 Free PMC article. Review. - The high-energy transition state of the glutamate transporter homologue GltPh.
Huysmans GHM, Ciftci D, Wang X, Blanchard SC, Boudker O. Huysmans GHM, et al. EMBO J. 2021 Jan 4;40(1):e105415. doi: 10.15252/embj.2020105415. Epub 2020 Nov 13. EMBO J. 2021. PMID: 33185289 Free PMC article. - Induced fit substrate binding to an archeal glutamate transporter homologue.
Ewers D, Becher T, Machtens JP, Weyand I, Fahlke C. Ewers D, et al. Proc Natl Acad Sci U S A. 2013 Jul 23;110(30):12486-91. doi: 10.1073/pnas.1300772110. Epub 2013 Jul 9. Proc Natl Acad Sci U S A. 2013. PMID: 23840066 Free PMC article. - Engineered Cross-Linking to Study the Pore Architecture of the CRAC Channel.
Ma G, He L, Jing J, Tan P, Huang Y, Zhou Y. Ma G, et al. Methods Mol Biol. 2018;1843:147-166. doi: 10.1007/978-1-4939-8704-7_13. Methods Mol Biol. 2018. PMID: 30203285 Free PMC article.
References
- Torres GE, Amara SG. Glutamate and monoamine transporters: New visions of form and function. Curr Opin Neurobiol. 2007;17:304–312. - PubMed
- Zerangue N, Kavanaugh MP. Flux coupling in a neuronal glutamate transporter. Nature. 1996;383:634–637. - PubMed
- Levy LM, et al. Inducible expression of the GLT-1 glutamate transporter in a CHO cell line selected for low endogenous glutamate uptake. FEBS Lett. 1998;422:339–342. - PubMed
- Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375:599–603. - PubMed
- Wadiche JI, Amara SG, Kavanaugh MP. Ion fluxes associated with excitatory amino acid transport. Neuron. 1995;15:721–728. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1U54GM087519-01A1/GM/NIGMS NIH HHS/United States
- MH080726/MH/NIMH NIH HHS/United States
- R01-GM086328/GM/NIGMS NIH HHS/United States
- U54 GM087519/GM/NIGMS NIH HHS/United States
- R01 MH080726/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources