Protein O-linked β-N-acetylglucosamine: a novel effector of cardiomyocyte metabolism and function - PubMed (original) (raw)
Review
Protein O-linked β-N-acetylglucosamine: a novel effector of cardiomyocyte metabolism and function
Victor M Darley-Usmar et al. J Mol Cell Cardiol. 2012 Mar.
Abstract
The post-translational modification of serine and threonine residues of nuclear and cytoplasmic proteins by the O-linked attachment of the monosaccharide β-N-acetyl-glucosamine (O-GlcNAc) is emerging as an important mechanism for the regulation of numerous biological processes critical for normal cell function. Active synthesis of O-GlcNAc is essential for cell viability and acute activation of pathways resulting in increased protein O-GlcNAc levels improves the tolerance of cells to a wide range of stress stimuli. Conversely sustained increases in O-GlcNAc levels have been implicated in numerous chronic disease states, especially as a pathogenic contributor to diabetic complications. There has been increasing interest in the role of O-GlcNAc in the heart and vascular system and acute activation of O-GlcNAc levels have been shown to reduce ischemia/reperfusion injury, attenuate vascular injury responses as well mediate some of the detrimental effects of diabetes and hypertension on cardiac and vascular function. Here we provide an overview of our current understanding of pathways regulating protein O-GlcNAcylation, summarize the different methodologies for identifying and characterizing O-GlcNAcylated proteins and subsequently focus on two emerging areas: 1) the role of O-GlcNAc as a potential regulator of cardiac metabolism and 2) the cross talk between O-GlcNAc and reactive oxygen species. This article is part of a Special Section entitled "Post-translational Modification."
Copyright © 2011 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosures: None
Figures
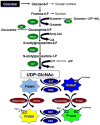
Figure 1. The hexosamine biosynthesis pathway (HBP) and protein O-GlcNAcylation
Glucose imported into the cells is rapidly phosphorylated to glucose-6-phosphate and converted to fructose-6-phosphate, which is subsequently metabolized to glucosamine-6-phosphate by L-glutamine-
D
-fructose 6-phosphate amidotransferase (GFAT). Synthesis of glucosamine-6-phosphate is dependent on availability of glutamine, which is formed by glutamine synthetase (GTS). Glucosamine-6-phosphate is subsequently metabolized by glucosamine 6-phosphate N-acetyltransferase (Emeg32) to N-acetylglucosamine-6-phosphate, which is converted to N-acetylglucosamine-1-phosphate by Phosphoacetylglucosamine mutase (Agm1) and the synthesis of UDP- uridine-diphosphate-N-acetylglucosamine (UDP-GlcNAc) is catalyzed by UDP-N-acetylglucosamine pyrophosphorylase (Uap1). Flux through the HBP can be increased with glucosamine, which is phosphorylated by hexokinase (HK) to form glucosamine 6-phosphate thereby bypassing GFAT. UDP-GlcNAc is the obligatory substrate for OGT (uridine-diphospho-N-acetylglucosamine: polypeptide β-N-acetylglucosaminyltransferase) leading to the formation of O-linked ß-N-acetylglucosamine (O-GlcNAc)-modified proteins. ß-N-acetylglucosaminidase (OGA) catalyzes the removal of O-GlcNAc from the proteins.
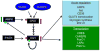
Figure 2. Acute and transcriptional regulation of cardiac metabolism mediated by the hexosamine biosynthesis pathway (HBP) and O-GlcNAc
Flux through the HBP is regulated by glutamine-
D
-fructose 6-phosphate amidotransferase (GFAT) and is dependent on the availability of glucose and glutamine. O-GlcNAc transferase (OGT) is subject to both O-GlcNAcylation (green circles) and insulin-mediated phosphorylation (red circles) both of which have been reported to increase its activity. AMPK, which is well recognized as a key regulator of cellular metabolism has been reported to be a target for O-GlcNAcylation and that this increases its activity; furthermore, GFAT has been shown to phosphorylated by AMPK also leading to increased activity. This provides a feed forward loop leading to further increase in OGT activity and subsequent increase in O-GlcNAcylation. Increases in O-GlcNAc levels can potentially acutely regulate cardiac metabolism, not only by modification of AMPK, but also by O-GlcNAcylation of other key mediators of carbohydrate and fatty acid metabolism. A number of transcription factors, which play critical role in the longer-term regulation of metabolism, are also known to be O-GlcNAcylated.
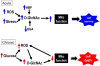
Figure 3. The intersection between O-GlcNAcylation, ROS and mitochondrial function
In response to an acute stress, including that induced by ROS, there is a rapid increase in cellular and potentially mitochondrial O-GlcNAcylation as a result of either an increased flux through the hexosamine biosynthesis pathway (HBP) and/or decreased O-GlcNAcase activity (OGA). This acute increase in O-GlcNAc levels has been shown to improve tolerance of mitochondria to oxidative stress and levels and attenuate loss of mitochondrial membrane potential leading to increased cell survival. Strategies designed to acutely augment this response have been shown to improve cell survival; conversely decreased O-GlcNAc levels lead to decreased cell survival. Chronic increases in cellular O-GlcNAc levels are typically associated with metabolic disease such as diabetes and the resulting hyperglycemia. Sustained increases in glucose levels can increase O-GlcNAc synthesis by directly increasing flux through the HBP as well as indirectly, as a result of hyperglycemia induced ROS production, which has also been shown to stimulate O-GlcNAc synthesis. Hyperglycemia is also known to lead to impaired mitochondrial function in part due to increased O-GlcNAcylation of mitochondrial proteins, which could in turn further increase ROS production and thereby in a feed forward manner continue to increase O-GlcNAc levels with even greater decline in mitochondria function. This O-GlcNAc mediated impairment of mitochondrial function, could then increase the vulnerability to additional stress thus leading to increased cell death. Indeed, targeted overexpression of mitochondrial OGT has been shown to lead to increased apoptosis.
Similar articles
- Protein O-GlcNAcylation: a new signaling paradigm for the cardiovascular system.
Laczy B, Hill BG, Wang K, Paterson AJ, White CR, Xing D, Chen YF, Darley-Usmar V, Oparil S, Chatham JC. Laczy B, et al. Am J Physiol Heart Circ Physiol. 2009 Jan;296(1):H13-28. doi: 10.1152/ajpheart.01056.2008. Epub 2008 Nov 21. Am J Physiol Heart Circ Physiol. 2009. PMID: 19028792 Free PMC article. - Post-translational protein modification by O-linked N-acetyl-glucosamine: its role in mediating the adverse effects of diabetes on the heart.
McLarty JL, Marsh SA, Chatham JC. McLarty JL, et al. Life Sci. 2013 Mar 28;92(11):621-7. doi: 10.1016/j.lfs.2012.08.006. Epub 2012 Aug 11. Life Sci. 2013. PMID: 22985933 Free PMC article. Review. - The role of protein O-linked beta-N-acetylglucosamine in mediating cardiac stress responses.
Chatham JC, Marchase RB. Chatham JC, et al. Biochim Biophys Acta. 2010 Feb;1800(2):57-66. doi: 10.1016/j.bbagen.2009.07.004. Epub 2009 Jul 14. Biochim Biophys Acta. 2010. PMID: 19607882 Free PMC article. Review. - The role of protein O-GlcNAcylation in diabetic cardiomyopathy.
Chatham JC, Wende AR. Chatham JC, et al. Biochem Soc Trans. 2024 Dec 19;52(6):2343-2358. doi: 10.1042/BST20240262. Biochem Soc Trans. 2024. PMID: 39601777 Review. - Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2.
Champattanachai V, Marchase RB, Chatham JC. Champattanachai V, et al. Am J Physiol Cell Physiol. 2008 Jun;294(6):C1509-20. doi: 10.1152/ajpcell.00456.2007. Epub 2008 Mar 26. Am J Physiol Cell Physiol. 2008. PMID: 18367586 Free PMC article.
Cited by
- The dual role of the hexosamine biosynthetic pathway in cardiac physiology and pathophysiology.
Cairns M, Joseph D, Essop MF. Cairns M, et al. Front Endocrinol (Lausanne). 2022 Oct 24;13:984342. doi: 10.3389/fendo.2022.984342. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36353238 Free PMC article. Review. - Protein O-GlcNAcylation and cardiovascular (patho)physiology.
Marsh SA, Collins HE, Chatham JC. Marsh SA, et al. J Biol Chem. 2014 Dec 12;289(50):34449-56. doi: 10.1074/jbc.R114.585984. Epub 2014 Oct 21. J Biol Chem. 2014. PMID: 25336635 Free PMC article. Review. - Basic Mechanisms of Diabetic Heart Disease.
Ritchie RH, Abel ED. Ritchie RH, et al. Circ Res. 2020 May 22;126(11):1501-1525. doi: 10.1161/CIRCRESAHA.120.315913. Epub 2020 May 21. Circ Res. 2020. PMID: 32437308 Free PMC article. Review. - Defining the Dynamic Regulation of O-GlcNAc Proteome in the Mouse Cortex---the O-GlcNAcylation of Synaptic and Trafficking Proteins Related to Neurodegenerative Diseases.
Huynh VN, Wang S, Ouyang X, Wani WY, Johnson MS, Chacko BK, Jegga AG, Qian WJ, Chatham JC, Darley-Usmar VM, Zhang J. Huynh VN, et al. Front Aging. 2021 Sep 29;2:757801. doi: 10.3389/fragi.2021.757801. eCollection 2021. Front Aging. 2021. PMID: 35822049 Free PMC article. - A Novel Glycoproteomics Workflow Reveals Dynamic O-GlcNAcylation of COPγ1 as a Candidate Regulator of Protein Trafficking.
Cox NJ, Luo PM, Smith TJ, Bisnett BJ, Soderblom EJ, Boyce M. Cox NJ, et al. Front Endocrinol (Lausanne). 2018 Oct 15;9:606. doi: 10.3389/fendo.2018.00606. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30459710 Free PMC article.
References
- Spiro RG. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12:43R–56R. - PubMed
- Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984;259:3308–17. - PubMed
- Hu P, Shimoji S, Hart GW. Site-specific interplay between O-GlcNAcylation and phosphorylation in cellular regulation. FEBS letters. 2010;584:2526–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 DK075867/DK/NIDDK NIH HHS/United States
- R01 DE020925/DE/NIDCR NIH HHS/United States
- ES10167/ES/NIEHS NIH HHS/United States
- R01 AA013395/AA/NIAAA NIH HHS/United States
- R01 HL079364/HL/NHLBI NIH HHS/United States
- R01 HL101192/HL/NHLBI NIH HHS/United States
- HL101192/HL/NHLBI NIH HHS/United States
- DK075867/DK/NIDDK NIH HHS/United States
- R01 ES010167/ES/NIEHS NIH HHS/United States
- DE020925/DE/NIDCR NIH HHS/United States
- AA13395/AA/NIAAA NIH HHS/United States
- HL079364/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources