Rapid automatic assessment of microvascular density in sidestream dark field images - PubMed (original) (raw)
Rapid automatic assessment of microvascular density in sidestream dark field images
Rick Bezemer et al. Med Biol Eng Comput. 2011 Nov.
Erratum in
- Med Biol Eng Comput. 2012 Oct;50(10):1115. Christiaan Boerma, E [corrected to Boerma, E Christiaan]
Abstract
The purpose of this study was to develop a rapid and fully automatic method for the assessment of microvascular density and perfusion in sidestream dark field (SDF) images. We modified algorithms previously developed by our group for microvascular density assessment and introduced a new method for microvascular perfusion assessment. To validate the new algorithm for microvascular density assessment, we reanalyzed a selection of SDF video clips (n = 325) from a study in intensive care patients and compared the results to (semi-)manually found microvascular densities. The method for microvascular perfusion assessment (temporal SDF image contrast analysis, tSICA) was tested in several video simulations and in one high quality SDF video clip where the microcirculation was imaged before and during circulatory arrest in a cardiac surgery patient. We found that the new method for microvascular density assessment was very rapid (<30 s/clip) and correlated excellently with (semi-)manually measured microvascular density. The new method for microvascular perfusion assessment (tSICA) was shown to be limited by high cell densities and velocities, which severely impedes the applicability of this method in real SDF images. Hence, here we present a validated method for rapid and fully automatic assessment of microvascular density in SDF images. The new method was shown to be much faster than the conventional (semi-)manual method. Due to current SDF imaging hardware limitations, we were not able to automatically detect microvascular perfusion.
Figures
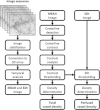
Fig. 1
A diagram schematically depicting the analysis steps required for microvascular density and perfusion assessment. First an image sequence is stabilized and converted to a 3D array. From the 3D array, two images are generated: (1) a time-averaged (MEAN) image; and (2) an image representing the temporal pixel intensity fluctuations, quantified as the standard deviation of intensities in time (SDt). Subsequently, the MEAN image is used to detect vessel centerlines and validate the centerlines based on contrast thresholding. Then, the SDt image can be used to classify a validated centerline as being perfused or not
Fig. 2
Correlation, linear regression (left), and Bland–Altman (right) analysis of manually determined total vessel length (TVD) versus fully automatically determined TVD in sidestream darkfield (SDF) video clips where the proportion of perfused vessels (PPV) was 100% (upper) and less than 100% (lower). The Bland–Altman plots show the TVD difference (i.e., the manually determined TVD minus the automatically determined TVD) versus the average TVD. For the video clips in which PPV = 100%, the 95% limits of agreement were −2.68 and 1.72 mm/mm2 and for the video clips in which PPV < 100%, the 95% limits of agreement were −2.36 and 2.26 mm/mm2. The new method adequately recovers TVD and is insensitive to vessel perfusion (i.e., good agreement for clips with PPV = 100% and PPV < 100%)
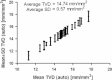
Fig. 3
The repeatability of the automatic measurement of total vessel density (TVD) in 25 video clips with normal perfusion that were highly stable (i.e., instability ≤5 pixels/s) over at least 1 s. The average measurement standard deviation (SD) is 0.57 mm/mm2 which is less than 4% of the average measured TVD of 14.74 mm/mm2
Fig. 4
Simulated vessels in ‘sidestream dark field (SDF) images’ (left column) and corresponding SDt images (right column) for different cell velocities (0 and 800 μm/s), cell densities (2 and 4 cells/100 μm2) and imaging rates (25 and 100 Hz). High cell density in combination with high cell velocity limits the detection of variations in pixel intensities (SDt) consequent to RBC passages, which might be improved by increasing the imaging rate
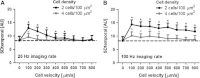
Fig. 5
Characterization of SDt as a function of cell velocity (0–800 μm/s), cell density (2 and 4 cells/100 μm2) and imaging rate (25 and 100 Hz; A and B, respectively). The SDt is low at zero flow, highest at low flow, and then decreases with increasing flow. *P < 0.05 versus cell velocity = 0 μm/s (i.e., no perfusion)
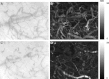
Fig. 6
Sidestream dark field (SDF) images before (A) and during C circulatory arrest and the corresponding SDt images (B and D, respectively). The SDt image at before circulatory arrest is clearly brighter (indicating flow) than the SDt image at stopped flow
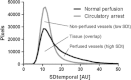
Fig. 7
SDt histograms before (black line) and during (gray line) circulatory arrest. The area under the histograms can be divided in three parts: first, the tissue (background) which was present both with and without perfusion; second, the perfused vessels which were only detected before cardiac arrest; and third, the non-perfused vessels which were only detected during circulatory arrest
Similar articles
- Cutaneous microcirculation in preterm neonates: comparison between sidestream dark field (SDF) and incident dark field (IDF) imaging.
van Elteren HA, Ince C, Tibboel D, Reiss IK, de Jonge RC. van Elteren HA, et al. J Clin Monit Comput. 2015 Oct;29(5):543-8. doi: 10.1007/s10877-015-9708-5. Epub 2015 May 29. J Clin Monit Comput. 2015. PMID: 26021740 Free PMC article. - An automated method for analysis of microcirculation videos for accurate assessment of tissue perfusion.
Demir SU, Hakimzadeh R, Hargraves RH, Ward KR, Myer EV, Najarian K. Demir SU, et al. BMC Med Imaging. 2012 Dec 21;12:37. doi: 10.1186/1471-2342-12-37. BMC Med Imaging. 2012. PMID: 23259402 Free PMC article. - Noninvasive assessment of the iridial microcirculation in rats using sidestream dark field imaging.
Cerny V, Zhou J, Kelly M, Alotibi I, Turek Z, Whynot S, Saleh IA, Lehmann C. Cerny V, et al. J Microsc. 2013 Feb;249(2):119-23. doi: 10.1111/jmi.12000. Epub 2012 Dec 20. J Microsc. 2013. PMID: 23277920 - Microvascular perfusion in cardiac arrest: a review of microcirculatory imaging studies.
Krupičková P, Mormanová Z, Bouček T, Belza T, Šmalcová J, Bělohlávek J. Krupičková P, et al. Perfusion. 2018 Jan;33(1):8-15. doi: 10.1177/0267659117723455. Epub 2017 Aug 16. Perfusion. 2018. PMID: 28812428 Review. - Monitoring the microcirculation in the critically ill patient: current methods and future approaches.
De Backer D, Ospina-Tascon G, Salgado D, Favory R, Creteur J, Vincent JL. De Backer D, et al. Intensive Care Med. 2010 Nov;36(11):1813-25. doi: 10.1007/s00134-010-2005-3. Epub 2010 Aug 6. Intensive Care Med. 2010. PMID: 20689916 Review.
Cited by
- Quantitative color analysis for capillaroscopy image segmentation.
Goffredo M, Schmid M, Conforto S, Amorosi B, D'Alessio T, Palma C. Goffredo M, et al. Med Biol Eng Comput. 2012 Jun;50(6):567-74. doi: 10.1007/s11517-012-0907-7. Epub 2012 Apr 25. Med Biol Eng Comput. 2012. PMID: 22532162 - Videomicroscopy as a tool for investigation of the microcirculation in the newborn.
Wright IM, Latter JL, Dyson RM, Levi CR, Clifton VL. Wright IM, et al. Physiol Rep. 2016 Oct;4(19):e12941. doi: 10.14814/phy2.12941. Physiol Rep. 2016. PMID: 27694527 Free PMC article. - Deep learning and computer vision techniques for microcirculation analysis: A review.
Helmy M, Truong TT, Jul E, Ferreira P. Helmy M, et al. Patterns (N Y). 2022 Dec 1;4(1):100641. doi: 10.1016/j.patter.2022.100641. eCollection 2023 Jan 13. Patterns (N Y). 2022. PMID: 36699745 Free PMC article. Review. - Assessing the Microcirculation With Handheld Vital Microscopy in Critically Ill Neonates and Children: Evolution of the Technique and Its Potential for Critical Care.
Erdem Ö, Ince C, Tibboel D, Kuiper JW. Erdem Ö, et al. Front Pediatr. 2019 Jul 9;7:273. doi: 10.3389/fped.2019.00273. eCollection 2019. Front Pediatr. 2019. PMID: 31338353 Free PMC article. Review. - Qualitative real-time analysis by nurses of sublingual microcirculation in intensive care unit: the MICRONURSE study.
Tanaka S, Harrois A, Nicolaï C, Flores M, Hamada S, Vicaut E, Duranteau J. Tanaka S, et al. Crit Care. 2015 Nov 6;19:388. doi: 10.1186/s13054-015-1106-3. Crit Care. 2015. PMID: 26542952 Free PMC article.
References
- Boerma EC, Koopmans M, Konijn A, Kaiferova K, Bakker AJ, Roon EN, Buter H, Bruins N, Egbers PH, Gerritsen RT, Koetsier PM, Kingma WP, Kuiper MA, Ince C. Effects of nitroglycerin on sublingual microcirculatory blood flow in patients with severe sepsis/septic shock after a strict resuscitation protocol: a double-blind randomized placebo controlled trial. Crit Care Med. 2010;38:93–100. doi: 10.1097/CCM.0b013e3181b02fc1. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources