The ageing systemic milieu negatively regulates neurogenesis and cognitive function - PubMed (original) (raw)
. 2011 Aug 31;477(7362):90-4.
doi: 10.1038/nature10357.
Jian Luo, Kira I Mosher, Bende Zou, Markus Britschgi, Gregor Bieri, Trisha M Stan, Nina Fainberg, Zhaoqing Ding, Alexander Eggel, Kurt M Lucin, Eva Czirr, Jeong-Soo Park, Sebastien Couillard-Després, Ludwig Aigner, Ge Li, Elaine R Peskind, Jeffrey A Kaye, Joseph F Quinn, Douglas R Galasko, Xinmin S Xie, Thomas A Rando, Tony Wyss-Coray
Affiliations
- PMID: 21886162
- PMCID: PMC3170097
- DOI: 10.1038/nature10357
The ageing systemic milieu negatively regulates neurogenesis and cognitive function
Saul A Villeda et al. Nature. 2011.
Abstract
In the central nervous system, ageing results in a precipitous decline in adult neural stem/progenitor cells and neurogenesis, with concomitant impairments in cognitive functions. Interestingly, such impairments can be ameliorated through systemic perturbations such as exercise. Here, using heterochronic parabiosis we show that blood-borne factors present in the systemic milieu can inhibit or promote adult neurogenesis in an age-dependent fashion in mice. Accordingly, exposing a young mouse to an old systemic environment or to plasma from old mice decreased synaptic plasticity, and impaired contextual fear conditioning and spatial learning and memory. We identify chemokines--including CCL11 (also known as eotaxin)--the plasma levels of which correlate with reduced neurogenesis in heterochronic parabionts and aged mice, and the levels of which are increased in the plasma and cerebrospinal fluid of healthy ageing humans. Lastly, increasing peripheral CCL11 chemokine levels in vivo in young mice decreased adult neurogenesis and impaired learning and memory. Together our data indicate that the decline in neurogenesis and cognitive impairments observed during ageing can be in part attributed to changes in blood-borne factors.
Conflict of interest statement
Competing Interests Statement: The authors declare that they have no competing financial interests.
Figures
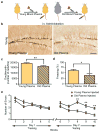
Figure 1. Heterochronic parabiosis alters neurogenesis in an age-dependent fashion
a, Schematic showing parabiotic pairings. b,e, Representative fields of Doublecortin (b) and BrdU (e) immunostaining of young (3–4 months; yellow) and old (18–20 months; gray) isochronic and heterochronic parabionts five weeks after parabiosis (arrowheads point to individual cells, scale bar: 100μm). c–f Quantification of neurogenesis (c,d) and proliferating cells (e,f) in the young (c,e; top) and old (d,f; bottom) DG after parabiosis. Data from 12 young isochronic, 10 young heterochronic, 6 old isochronic and 12 old heterochronic parabionts. g,h, Population spike amplitude (PSA) was recorded from DG of young parabionts. Representative electrophysiological profiles (g) and LTP levels (h) are shown for young heterochronic and isochronic parabionts. Data from 4–5 mice per group. All data represented as Mean + SEM; *P<0.05; **P<0.01 t-test.
Figure 2. Factors from an old systemic environment decrease neurogenesis and impair learning and memory
a, Schematic of young (3–4 months) or old (18–22 months) plasma extraction and intravenous injection into young (3 months) adult mice. b, Representative field of Doublecortin immunostaining of young adult mice after plasma injection treatment four times over ten days (scale bar: 100μm). c, Quantification of neurogenesis in the young DG after plasma injection. Data from 8 young plasma and 7 old plasma injected mice. d,e Hippocampal learning and memory assessed by contextual fear conditioning (d) and RAWM (e) paradigms in young adult mice after young or old plasma injections nine times over 24 days. d, Percent freezing time 24 hours after training. Data from 8 mice per group. e, Number of entry arm errors prior to finding platform. Data from 12 mice per group. All data represented as Mean ± SEM; *P< 0.05; **P< 0.01; t-test (c,d), repeated measures ANOVA, Bonferroni post-hoc test (e).
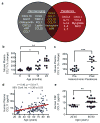
Figure 3. Systemic chemokine levels increase during aging and heterochronic parabiosis and correlate with decreased neurogenesis
a, Venn diagram of results from aging and parabiosis proteomic screens. Seventeen age-related plasma factors correlated strongest with decreased neurogenesis in gray, fifteen plasma factors increased between young isochronic and young heterochronic parabionts in red, and six factors elevated in both screens in brown intersection. Data from 5–6 animals per age group. b,c Changes in plasma concentrations of CCL11 with age (b) and young heterochronic parabionts pre- and post- parabiotic pairing (c). d,e Changes in plasma (d) and CSF (e) concentrations of CCL11 with age in healthy human subjects. All data represented as dot plots with mean; *P< 0.05; **P< 0.01; ***P< 0.001 t-test (c,e), ANOVA, Tukey’s post-hoc test (a,b), and Mann-Whitney U Test (d).
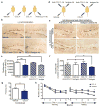
Figure 4. Systemic exposure to CCL11 inhibits neurogenesis and impairs learning and memory
a, Schematic of young (3–4 months) mice injected intraperitoneally with CCL11 or vehicle, and in combination with anti-CCL11 neutralizing or isotype control antibody. b, Representative field of Dcx-positive cells for each treatment group (n = 6–10 mice) treated four times over ten days. (scale bar: 100μm). c, Quantification of neurogenesis in the DG after treatment. d, Schematic of young adult mice given unilateral stereotaxic injections of anti-CCL11 neutralizing or isotype control antibody followed by systemic injections with either recombinant CCL11 or PBS (vehicle). e, Representative field of Dcx-positive cells in adjacent sides of the DG for each treatment group (n = 3–11 mice) f, Quantification of neurogenesis in the DG after systemic and stereotaxic treatment. g,h, Learning and memory assessed by contextual fear conditioning (g) and RAWM (h) paradigms in young adult mice injected with CCL11 or vehicle every three days for five weeks (n = 12–16 mice per group). All data represented as Mean ± SEM; *P< 0.05; **P<0.01; ANOVA, Dunnet’s or Tukey’s post-hoc test (c,f); repeated measures ANOVA, Bonferroni post-hoc test (k).
Comment in
- Ageing: Blood ties.
Ransohoff RM. Ransohoff RM. Nature. 2011 Aug 31;477(7362):41-2. doi: 10.1038/477041a. Nature. 2011. PMID: 21886154 No abstract available. - Ageing: ageing, it's in the blood.
Yates D. Yates D. Nat Rev Neurosci. 2011 Sep 20;12(10):547. doi: 10.1038/nrn3116. Nat Rev Neurosci. 2011. PMID: 21931332 No abstract available.
Similar articles
- Overcoming the aging systemic milieu to restore neural stem cell function.
Mendelsohn AR, Larrick JW. Mendelsohn AR, et al. Rejuvenation Res. 2011 Dec;14(6):681-4. doi: 10.1089/rej.2011.1301. Rejuvenation Res. 2011. PMID: 22175510 - β2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis.
Smith LK, He Y, Park JS, Bieri G, Snethlage CE, Lin K, Gontier G, Wabl R, Plambeck KE, Udeochu J, Wheatley EG, Bouchard J, Eggel A, Narasimha R, Grant JL, Luo J, Wyss-Coray T, Villeda SA. Smith LK, et al. Nat Med. 2015 Aug;21(8):932-7. doi: 10.1038/nm.3898. Epub 2015 Jul 6. Nat Med. 2015. PMID: 26147761 Free PMC article. - Young bone marrow transplantation preserves learning and memory in old mice.
Das MM, Godoy M, Chen S, Moser VA, Avalos P, Roxas KM, Dang I, Yáñez A, Zhang W, Bresee C, Arditi M, Liu GY, Svendsen CN, Goodridge HS. Das MM, et al. Commun Biol. 2019 Feb 20;2:73. doi: 10.1038/s42003-019-0298-5. eCollection 2019. Commun Biol. 2019. PMID: 30820468 Free PMC article. - The systemic environment: at the interface of aging and adult neurogenesis.
Smith LK, White CW 3rd, Villeda SA. Smith LK, et al. Cell Tissue Res. 2018 Jan;371(1):105-113. doi: 10.1007/s00441-017-2715-8. Epub 2017 Nov 9. Cell Tissue Res. 2018. PMID: 29124393 Free PMC article. Review. - An emerging role for eotaxins in neurodegenerative disease.
Huber AK, Giles DA, Segal BM, Irani DN. Huber AK, et al. Clin Immunol. 2018 Apr;189:29-33. doi: 10.1016/j.clim.2016.09.010. Epub 2016 Sep 21. Clin Immunol. 2018. PMID: 27664933 Review.
Cited by
- Plasma from healthy donors protects blood-brain barrier integrity via FGF21 and improves the recovery in a mouse model of cerebral ischaemia.
Mamtilahun M, Jiang L, Song Y, Shi X, Liu C, Jiang Y, Deng L, Zheng H, Shen H, Li Y, Zhang Z, Wang Y, Tang Y, Yang GY. Mamtilahun M, et al. Stroke Vasc Neurol. 2021 Dec;6(4):561-571. doi: 10.1136/svn-2020-000774. Epub 2021 Mar 30. Stroke Vasc Neurol. 2021. PMID: 33785536 Free PMC article. - Systemic regulation of the age-related decline of pancreatic β-cell replication.
Salpeter SJ, Khalaileh A, Weinberg-Corem N, Ziv O, Glaser B, Dor Y. Salpeter SJ, et al. Diabetes. 2013 Aug;62(8):2843-8. doi: 10.2337/db13-0160. Epub 2013 Apr 29. Diabetes. 2013. PMID: 23630298 Free PMC article. - Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH.
Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D. Zhang G, et al. Nature. 2013 May 9;497(7448):211-6. doi: 10.1038/nature12143. Epub 2013 May 1. Nature. 2013. PMID: 23636330 Free PMC article. - Association of accelerated long-term forgetting and senescence-related blood-borne factors in asymptomatic individuals from families with autosomal dominant Alzheimer's disease.
Yang J, Kong C, Jia L, Li T, Quan M, Li Y, Lyu D, Li F, Jin H, Li Y, Wang Q, Jia J. Yang J, et al. Alzheimers Res Ther. 2021 May 27;13(1):107. doi: 10.1186/s13195-021-00845-0. Alzheimers Res Ther. 2021. PMID: 34044860 Free PMC article. - Ageing of the heart reversed by youthful systemic factors!
Brack AS. Brack AS. EMBO J. 2013 Aug 14;32(16):2189-90. doi: 10.1038/emboj.2013.162. Epub 2013 Jul 16. EMBO J. 2013. PMID: 23860129 Free PMC article.
References
- Gage FH. Mammalian neural stem cells. Science. 2000;287 (5457):1433–1438. - PubMed
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41 (5):683–686. - PubMed
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132 (4):645–660. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007290/AI/NIAID NIH HHS/United States
- F31 AG034045-03/AG/NIA NIH HHS/United States
- R01 MH078194/MH/NIMH NIH HHS/United States
- DP1 OD000392-02/OD/NIH HHS/United States
- F31 AG034045-02/AG/NIA NIH HHS/United States
- DP1 OD000392-04/OD/NIH HHS/United States
- DP1 OD000392-01/OD/NIH HHS/United States
- DP1 OD000392-05/OD/NIH HHS/United States
- F31 AG034045-01/AG/NIA NIH HHS/United States
- R01 AG027505-02/AG/NIA NIH HHS/United States
- 1 F31 AG034045-01/AG/NIA NIH HHS/United States
- R01AG027505/AG/NIA NIH HHS/United States
- R01 AG027505-01A1/AG/NIA NIH HHS/United States
- P30AG08017/AG/NIA NIH HHS/United States
- DP1 OD000392/OD/NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
- R01 AG027505-05/AG/NIA NIH HHS/United States
- R01 AG027505/AG/NIA NIH HHS/United States
- R01 AR056849/AR/NIAMS NIH HHS/United States
- 1 F31 NS066676-01A1/NS/NINDS NIH HHS/United States
- P30 AG008017/AG/NIA NIH HHS/United States
- DP1 OD000392-03/OD/NIH HHS/United States
- R01 AG027505-04/AG/NIA NIH HHS/United States
- F31 NS066676/NS/NINDS NIH HHS/United States
- R01 AG027505-03/AG/NIA NIH HHS/United States
- F31 AG034045/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases