Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia - PubMed (original) (raw)
. 2011 Sep 4;43(10):1012-7.
doi: 10.1038/ng.913.
Chan-Eng Chong, Catherine L Carmichael, Ella J Wilkins, Peter J Brautigan, Xiao-Chun Li, Milena Babic, Ming Lin, Amandine Carmagnac, Young K Lee, Chung H Kok, Lucia Gagliardi, Kathryn L Friend, Paul G Ekert, Carolyn M Butcher, Anna L Brown, Ian D Lewis, L Bik To, Andrew E Timms, Jan Storek, Sarah Moore, Meryl Altree, Robert Escher, Peter G Bardy, Graeme K Suthers, Richard J D'Andrea, Marshall S Horwitz, Hamish S Scott
Affiliations
- PMID: 21892162
- PMCID: PMC3184204
- DOI: 10.1038/ng.913
Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia
Christopher N Hahn et al. Nat Genet. 2011.
Abstract
We report the discovery of GATA2 as a new myelodysplastic syndrome (MDS)-acute myeloid leukemia (AML) predisposition gene. We found the same, previously unidentified heterozygous c.1061C>T (p.Thr354Met) missense mutation in the GATA2 transcription factor gene segregating with the multigenerational transmission of MDS-AML in three families and a GATA2 c.1063_1065delACA (p.Thr355del) mutation at an adjacent codon in a fourth MDS family. The resulting alterations reside within the second zinc finger of GATA2, which mediates DNA-binding and protein-protein interactions. We show differential effects of the mutations on the transactivation of target genes, cellular differentiation, apoptosis and global gene expression. Identification of such predisposing genes to familial forms of MDS and AML is critical for more effective diagnosis and prognosis, counseling, selection of related bone marrow transplant donors and development of therapies.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Figures
Figure 1. Identification of novel germline p.Thr354Met and p.Thr355del variants in the highly conserved zinc finger 2 domain of GATA2 that is associated with MDS-AML
a. Pedigrees containing the p.Thr354Met and p.Thr355del variants. One family from Australia (Pedigree 1) and two from the USA (Pedigrees 2 and 3) display the p.Thr354Met variant segregating with MDS-AML, and one USA family (Pedigree 4) contains a p.Thr355del variant that segregates with MDS. The genotype of tested individuals is shown; T354, (Thr354/Thr354); T354M, (Thr354/Met354). b. Domain structure of GATA2 showing positions of mutations. The positions of the p.Thr354Met, p.Thr355del, AML-M5 (green) and CML BC (black) mutations are shown with respect to zinc finger (ZF) 1 and 2, transactivation domain (TA) and nuclear localization signal (NLS). c. Zinc finger 2 (ZF2) domain of GATA2 and GATA3 contains mutations associated with leukemia and breast cancer. The primary sequence is that of human GATA2 with the two alternative residues in GATA3 ZF2 shown (light grey with black letters). The position of p.Thr354Met and p.Thr355del is highlighted along with mutations found in GATA2 in AML-M5 (green) and CML BC (black), and in GATA3 in breast cancer (summarized in ) (mutated residues in the corresponding GATA3 ZF2; grey with white letters).
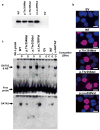
Figure 2. Subcellular localisation and DNA binding properties of GATA2 WT and mutants
HEK293 cells were transiently transfected with EV (pCMV-XL6 empty vector), WT, p.Thr354Met, p.Thr355del or p.Leu359Val and harvested after 24 h. a. Western blot analysis of GATA2 expression in nuclear lysates. Nuclear lysates were prepared and western blots performed, probing for GATA2. b. Cells were stained for GATA2 (red) and DAPI (blue). Scale bars, 10 μm. c. Electromobility shift assay (EMSA) of GATA2 WT and mutants. Nuclear lysates were prepared and bound to the TCRδ enhancer (contains GATA binding site) oligonucleotide in the absence or presence of 200-fold unlabeled competitor oligonucleotide (D, human TCRδ enhancer; C, GATA consensus; G, GM-CSF promoter). The probes were visualised using chemiluminescence (top panel). Note, GATA2 & NS relates to a band that contains both GATA2 and a non-specific (NS) protein. To visualise GATA2 alone, an EMSA-western blot was performed probing with polyclonal α-GATA2 antibody (bottom panel), showing the level of binding of GATA2 WT and mutants. A neutralizing α-GATA2 antibody in the far right lane removes GATA2, but not the non-specific binding protein (NS) (top panel), and the specificity of GATA2 is confirmed in the bottom panel.
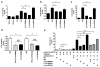
Figure 3. p.Thr354Met and p.Thr355del cause altered transactivation via target GATA2 response elements
p.Thr354Met and p.Thr355del act as a loss-of-function mutations on GATA2 target promoter and enhancer elements. HEK293 cells were cotransfected with 1) GATA2-responsive CD34 (mut – CD34 enhancer with GATA binding sites mutated) (a) and RUNX1 (b) enhancer elements linked to a LUC reporter, and 2) GATA2 (WT, p.Thr354Met, p.Thr355del or p.Leu359Val) expression constructs or pCMV6-XL6 empty vector (EV). Similarly, Cos-7 cells were cotransfected using LYL1 promoter LUC as reporter (c). After 20 h, cells were harvested and luciferase assays performed and plotted as fold (mean ± s.e.m.) compared to EV control. Pairwise comparisons are shown (*p<0.05, n = 3). d. p.Thr354Met and p.Thr355del act as dominant negative mutations over WT GATA2. HEK293 cells were cotransfected with: 1) CD34 enhancer-LUC reporter, and equivalent mole ratios of 2) WT to 3) p.Thr354Met or p.Thr355del. After 20 h, cells were harvested and luciferase assays performed. Pairwise comparisons are shown (*p<0.05; NS -not significant, n=3). e. p.Thr354Met has reduced ability to co-activate the CSF1R (M-CSF-R) promoter with PU.1. Cos-7 cells were cotransfected with 1) CSF1R promoter-LUC reporter, 2) PU.1 expression construct, and 3) WT, p.Thr354Met, p.Thr355del or p.Leu359Val expression constructs or EV. After 20 h, luciferase assays were performed and plotted as fold compared to EV. Pairwise comparisons are shown (*p<0.05, compared to WT plus PU.1; **p<0.05 compared to WT plus PU.1, but not significant when compared to p.Thr354Met or p.Thr355del plus PU.1, respectively). In all comparisons, a Student’s t-test was used.
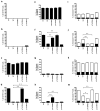
Figure 4. p.Thr354Met inhibits differentiation and apoptosis while allowing accumulation of cells in the presence of ATRA-induced differentiation
HL-60 cells carrying stably transduced 4HT-regulatable GATA2 (WT, p.Thr354Met, p.Thr355del and p.Leu359Val) or EV were treated with or without 30 nM 4HT for 24 h and then with or without 2 μM ATRA for 6 days. a–d. Differentiation of HL-60 cells into granulocytes. Differentiation was measured by FACS analysis for percentage of CD11b positive cells (mean ± s.e.m.) (see also Supplementary Fig. 7b). e–h. Cell numbers following differentiation. Cells were counted after 6 days (mean ± s.e.m.). i–m. Apoptosis following differentiation with ATRA. Cells were FACS analysed following staining with FITC anti-Annexin V and propidium iodide (PI). Annexin V+, PI− (black) or Annexin V+, PI+ (white). Indicative FACS plots (Supplementary Fig. 7c). a,e,i. −4HT, −ATRA; b,f,j. +4HT, −ATRA; c,g,k. −4HT, +ATRA; d,h,m. +4HT, +ATRA.(*p<0.05; **p<0.01, compared to WT). In all comparisons, a Student’s t-test was used.
Comment in
- GATA2 mutations lead to MDS and AML.
Hyde RK, Liu PP. Hyde RK, et al. Nat Genet. 2011 Sep 28;43(10):926-7. doi: 10.1038/ng.949. Nat Genet. 2011. PMID: 21956389 No abstract available.
Similar articles
- Loss-of-function germline GATA2 mutations in patients with MDS/AML or MonoMAC syndrome and primary lymphedema reveal a key role for GATA2 in the lymphatic vasculature.
Kazenwadel J, Secker GA, Liu YJ, Rosenfeld JA, Wildin RS, Cuellar-Rodriguez J, Hsu AP, Dyack S, Fernandez CV, Chong CE, Babic M, Bardy PG, Shimamura A, Zhang MY, Walsh T, Holland SM, Hickstein DD, Horwitz MS, Hahn CN, Scott HS, Harvey NL. Kazenwadel J, et al. Blood. 2012 Feb 2;119(5):1283-91. doi: 10.1182/blood-2011-08-374363. Epub 2011 Dec 6. Blood. 2012. PMID: 22147895 Free PMC article. - Heritable GATA2 mutations associated with familial AML-MDS: a case report and review of literature.
Gao J, Gentzler RD, Timms AE, Horwitz MS, Frankfurt O, Altman JK, Peterson LC. Gao J, et al. J Hematol Oncol. 2014 Apr 22;7:36. doi: 10.1186/1756-8722-7-36. J Hematol Oncol. 2014. PMID: 24754962 Free PMC article. Review. - GATA2 mutations lead to MDS and AML.
Hyde RK, Liu PP. Hyde RK, et al. Nat Genet. 2011 Sep 28;43(10):926-7. doi: 10.1038/ng.949. Nat Genet. 2011. PMID: 21956389 No abstract available. - Germ-line GATA2 p.THR354MET mutation in familial myelodysplastic syndrome with acquired monosomy 7 and ASXL1 mutation demonstrating rapid onset and poor survival.
Bödör C, Renneville A, Smith M, Charazac A, Iqbal S, Etancelin P, Cavenagh J, Barnett MJ, Kramarzová K, Krishnan B, Matolcsy A, Preudhomme C, Fitzgibbon J, Owen C. Bödör C, et al. Haematologica. 2012 Jun;97(6):890-4. doi: 10.3324/haematol.2011.054361. Epub 2012 Jan 22. Haematologica. 2012. PMID: 22271902 Free PMC article. - Quantitative and qualitative impairments in GATA2 and myeloid neoplasms.
Shimizu R, Yamamoto M. Shimizu R, et al. IUBMB Life. 2020 Jan;72(1):142-150. doi: 10.1002/iub.2188. Epub 2019 Nov 1. IUBMB Life. 2020. PMID: 31675473 Review.
Cited by
- GATA2 deficiency and hemophagocytic lymphohistiocytosis (HLH): a systematic review of reported cases.
Rukerd MRZ, Mirkamali H, Nakhaie M, Alizadeh SD. Rukerd MRZ, et al. BMC Infect Dis. 2024 Nov 4;24(1):1239. doi: 10.1186/s12879-024-10145-1. BMC Infect Dis. 2024. PMID: 39497062 Free PMC article. - Immunohistochemical Analysis of GATA2 Expression in Endometrium and its Relationship with Hormone Receptor Expression in Benign and Premalignant Endometrial Disorders.
Keske A, Polaki US, Matson DR. Keske A, et al. Reprod Sci. 2024 Dec;31(12):3880-3891. doi: 10.1007/s43032-024-01730-5. Epub 2024 Oct 23. Reprod Sci. 2024. PMID: 39443360 Free PMC article. - High frequency of GATA2 variants in patients with pulmonary fungal disease without immunocompromised risk factors: a retrospective study.
Zhuansun Y, He P, Du Y, Lin L, Chen R, Li J. Zhuansun Y, et al. J Thorac Dis. 2024 Aug 31;16(8):5180-5189. doi: 10.21037/jtd-24-583. Epub 2024 Aug 6. J Thorac Dis. 2024. PMID: 39268106 Free PMC article. - Molecular pathophysiology of germline mutations in acute myeloid leukemia.
Nagata Y. Nagata Y. Int J Hematol. 2024 Oct;120(4):417-426. doi: 10.1007/s12185-024-03824-x. Epub 2024 Aug 16. Int J Hematol. 2024. PMID: 39150677 Review. - Oncogenic Enhancers in Leukemia.
Mulet-Lazaro R, Delwel R. Mulet-Lazaro R, et al. Blood Cancer Discov. 2024 Sep 3;5(5):303-317. doi: 10.1158/2643-3230.BCD-23-0211. Blood Cancer Discov. 2024. PMID: 39093124 Review.
References
- Vardiman JW, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. - PubMed
- Barzi A, Sekeres MA. Myelodysplastic syndromes: a practical approach to diagnosis and treatment. Cleve Clin J Med. 2010;77:37–44. - PubMed
- Owen C, Barnett M, Fitzgibbon J. Familial myelodysplasia and acute myeloid leukaemia--a review. Br J Haematol. 2008;140:123–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous