Antidiabetic actions of a non-agonist PPARγ ligand blocking Cdk5-mediated phosphorylation - PubMed (original) (raw)
. 2011 Sep 4;477(7365):477-81.
doi: 10.1038/nature10383.
Alexander S Banks, Theodore M Kamenecka, Scott A Busby, Michael J Chalmers, Naresh Kumar, Dana S Kuruvilla, Youseung Shin, Yuanjun He, John B Bruning, David P Marciano, Michael D Cameron, Dina Laznik, Michael J Jurczak, Stephan C Schürer, Dušica Vidović, Gerald I Shulman, Bruce M Spiegelman, Patrick R Griffin
Affiliations
- PMID: 21892191
- PMCID: PMC3179551
- DOI: 10.1038/nature10383
Antidiabetic actions of a non-agonist PPARγ ligand blocking Cdk5-mediated phosphorylation
Jang Hyun Choi et al. Nature. 2011.
Abstract
PPARγ is the functioning receptor for the thiazolidinedione (TZD) class of antidiabetes drugs including rosiglitazone and pioglitazone. These drugs are full classical agonists for this nuclear receptor, but recent data have shown that many PPARγ-based drugs have a separate biochemical activity, blocking the obesity-linked phosphorylation of PPARγ by Cdk5. Here we describe novel synthetic compounds that have a unique mode of binding to PPARγ, completely lack classical transcriptional agonism and block the Cdk5-mediated phosphorylation in cultured adipocytes and in insulin-resistant mice. Moreover, one such compound, SR1664, has potent antidiabetic activity while not causing the fluid retention and weight gain that are serious side effects of many of the PPARγ drugs. Unlike TZDs, SR1664 also does not interfere with bone formation in culture. These data illustrate that new classes of antidiabetes drugs can be developed by specifically targeting the Cdk5-mediated phosphorylation of PPARγ.
© 2011 Macmillan Publishers Limited. All rights reserved
Conflict of interest statement
Competing interest statement: The authors declare no competing financial interests.
Figures
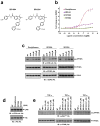
Figure 1. Novel PPARγ ligands lack classical agonism, block phosporylation at Ser273
a, Chemical structures of SR1664 and SR1824. b, Transcriptional activity of a PPAR-derived reporter gene in COS-1 cells following treatment with rosiglitazone, SR1664 or SR1824 (n=3). c and d, In vitro Cdk5 assay with rosiglitazone, SR1664 or SR1824 with PPARγ or Rb substrates. e, TNF-α-induced phosphorylation of PPARγ in differentiated PPARγ KO MEFs expressing PPARγWT treated with rosiglitazone, SR1664 or SR1824. Error bars are s.e.m.
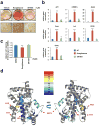
Figure 2. Structural and in vitro functional analysis of SR1664
a, Lipid accumulation in differentiated 3T3-L1 cells treated with rosiglitazone or SR1664 following Oil-Red-O staining. b, Expression of adipocyte-enriched genes in these cells was analyzed by qPCR (n=3). c, Mineralization of MC3T3-E1 osteoblast cells as determined by Alizarin Red-S. Error bars are s.e.m.; *p<0.05, **p<0.01, ***p<0.001, n.s.; not significant. NT, no treatment. d, Overlay of differential HDX data onto the docking model of 2hfp bound to SR1664 (see Supplemental Fig. 3). This overlay depicts the difference in HDX between ligand-free and SR1664 bound PPARγ LBD. Perturbation data are color coded and plotted onto the backbone of the PDB file according to the key. Observed changes in HDX were statistically significant (p<0.05) in a two tailed t-test (n=3).
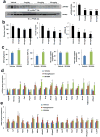
Figure 3. Anti-diabetic activity of SR1664 in high-fat diet (HFD) mice
a, Dose-dependent inhibition of phosphorylation of PPARγ by SR1664 in white adipose tissue (WAT). Quantification of PPARγ phosphorylation compared to total PPARγ (right). b, Ad libitum fed glucose (_p_=0.062 at 10mg/kg), insulin and HOMA-IR in HFD mice. c, Glucose infusion rate (GIR), suppression of hepatic glucose production (HGP), whole body glucose disposal and WAT 2-deoxyglucose tracer uptake during hyperinsulinemic-euglycemic clamps. d, Expression of a gene set regulated by PPARγ phosphorylation in WAT. e, Expression of an agonist gene set (see Methods) in WAT. Error bars are s.e.m.; *p<0.05, **p<0.01.
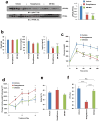
Figure 4. SR1664 has potent anti-diabetic activity and does not promote fluid retention in ob/ob mice
a, Phosphorylation of PPARγ in WAT (left). Quantification of PPARγ phosphorylation compared to total PPARγ (right). b and c, Fasting body weight, blood glucose and insulin levels prior to glucose-tolerance tests (GTT) in ob/ob mice treated with vehicle, rosiglitazone or SR1664 (n=8). Whole-body weight (d) and fat change (e) with continued drug administration following the GTT. f, Packed cell volume (PCV) in whole blood from ob/ob mice treated with vehicle, rosiglitazone or SR1664. Error bars are s.e.m.; *p<0.05, **p<0.01, ***p<0.001. n.s.; not significant.
Comment in
- Diabetes: T2DM-PPARγ ligands without the adverse effects?
Wilson C. Wilson C. Nat Rev Endocrinol. 2011 Sep 27;7(11):630. doi: 10.1038/nrendo.2011.167. Nat Rev Endocrinol. 2011. PMID: 21946892 No abstract available. - Diabetes: Safer PPARγ-targeted drugs on the horizon?
Crunkhorn S. Crunkhorn S. Nat Rev Drug Discov. 2011 Oct 14;10(11):814. doi: 10.1038/nrd3587. Nat Rev Drug Discov. 2011. PMID: 21997749 No abstract available.
Similar articles
- A novel non-agonist peroxisome proliferator-activated receptor γ (PPARγ) ligand UHC1 blocks PPARγ phosphorylation by cyclin-dependent kinase 5 (CDK5) and improves insulin sensitivity.
Choi SS, Kim ES, Koh M, Lee SJ, Lim D, Yang YR, Jang HJ, Seo KA, Min SH, Lee IH, Park SB, Suh PG, Choi JH. Choi SS, et al. J Biol Chem. 2014 Sep 19;289(38):26618-26629. doi: 10.1074/jbc.M114.566794. Epub 2014 Aug 6. J Biol Chem. 2014. PMID: 25100724 Free PMC article. - Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5.
Choi JH, Banks AS, Estall JL, Kajimura S, Boström P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Blüher M, Griffin PR, Spiegelman BM. Choi JH, et al. Nature. 2010 Jul 22;466(7305):451-6. doi: 10.1038/nature09291. Nature. 2010. PMID: 20651683 Free PMC article. - GQ-16, a novel peroxisome proliferator-activated receptor γ (PPARγ) ligand, promotes insulin sensitization without weight gain.
Amato AA, Rajagopalan S, Lin JZ, Carvalho BM, Figueira AC, Lu J, Ayers SD, Mottin M, Silveira RL, Souza PC, Mourão RH, Saad MJ, Togashi M, Simeoni LA, Abdalla DS, Skaf MS, Polikparpov I, Lima MC, Galdino SL, Brennan RG, Baxter JD, Pitta IR, Webb P, Phillips KJ, Neves FA. Amato AA, et al. J Biol Chem. 2012 Aug 10;287(33):28169-79. doi: 10.1074/jbc.M111.332106. Epub 2012 May 14. J Biol Chem. 2012. PMID: 22584573 Free PMC article. - Potent Anti-Diabetic Actions of a Novel Non-Agonist PPARγ Ligand that Blocks Cdk5-Mediated Phosphorylation.
Kamenecka TM, Busby SA, Kumar N, Choi JH, Banks AS, Vidovic D, Cameron MD, Schurer SC, Mercer BA, Hodder P, Spiegelman BM, Griffin PR. Kamenecka TM, et al. 2011 Jul 5 [updated 2013 Mar 7]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. 2011 Jul 5 [updated 2013 Mar 7]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. PMID: 23762958 Free Books & Documents. Review. - Peroxisome Proliferator-Activated Receptor γ (PPARγ) and Ligand Choreography: Newcomers Take the Stage.
Garcia-Vallvé S, Guasch L, Tomas-Hernández S, del Bas JM, Ollendorff V, Arola L, Pujadas G, Mulero M. Garcia-Vallvé S, et al. J Med Chem. 2015 Jul 23;58(14):5381-94. doi: 10.1021/jm501155f. Epub 2015 Apr 9. J Med Chem. 2015. PMID: 25734377 Review.
Cited by
- Leptin revisited: its mechanism of action and potential for treating diabetes.
Coppari R, Bjørbæk C. Coppari R, et al. Nat Rev Drug Discov. 2012 Sep;11(9):692-708. doi: 10.1038/nrd3757. Nat Rev Drug Discov. 2012. PMID: 22935803 Free PMC article. Review. - Selective targeting of PPARγ by the natural product chelerythrine with a unique binding mode and improved antidiabetic potency.
Zheng W, Qiu L, Wang R, Feng X, Han Y, Zhu Y, Chen D, Liu Y, Jin L, Li Y. Zheng W, et al. Sci Rep. 2015 Jul 17;5:12222. doi: 10.1038/srep12222. Sci Rep. 2015. PMID: 26183621 Free PMC article. - The E3 ubiquitin ligase TRIM25 regulates adipocyte differentiation via proteasome-mediated degradation of PPARγ.
Lee JM, Choi SS, Lee YH, Khim KW, Yoon S, Kim BG, Nam D, Suh PG, Myung K, Choi JH. Lee JM, et al. Exp Mol Med. 2018 Oct 15;50(10):1-11. doi: 10.1038/s12276-018-0162-6. Exp Mol Med. 2018. PMID: 30323259 Free PMC article. - Peroxisome proliferator-activated receptor gamma agonists in the prevention and treatment of murine systemic lupus erythematosus.
Aprahamian TR, Bonegio RG, Weitzner Z, Gharakhanian R, Rifkin IR. Aprahamian TR, et al. Immunology. 2014 Jul;142(3):363-73. doi: 10.1111/imm.12256. Immunology. 2014. PMID: 24456224 Free PMC article. - LXRα Phosphorylation in Cardiometabolic Disease: Insight From Mouse Models.
Voisin M, Gage MC, Becares N, Shrestha E, Fisher EA, Pineda-Torra I, Garabedian MJ. Voisin M, et al. Endocrinology. 2020 Jul 1;161(7):bqaa089. doi: 10.1210/endocr/bqaa089. Endocrinology. 2020. PMID: 32496563 Free PMC article. Review.
References
- Lehmann JM, et al. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) The Journal of biological chemistry. 1995;270:12953–12956. - PubMed
- Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. - PubMed
- Willson TM, Lambert MH, Kliewer SA. Peroxisome proliferator-activated receptor gamma and metabolic disease. Annu Rev Biochem. 2001;70:341–367. - PubMed
- Forman BM, et al. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 MH074404-01/MH/NIMH NIH HHS/United States
- 1RC4DK090861/DK/NIDDK NIH HHS/United States
- U54-MH074404/MH/NIMH NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- U24 DK059635/DK/NIDDK NIH HHS/United States
- R01 DK040936/DK/NIDDK NIH HHS/United States
- R01 GM084041-03/GM/NIGMS NIH HHS/United States
- DK31405/DK/NIDDK NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- R37 DK031405/DK/NIDDK NIH HHS/United States
- R01 GM084041/GM/NIGMS NIH HHS/United States
- U54 MH074404/MH/NIMH NIH HHS/United States
- RC4 DK090861/DK/NIDDK NIH HHS/United States
- RC4 DK090861-01/DK/NIDDK NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- R37 DK031405-30/DK/NIDDK NIH HHS/United States
- R01 DK031405/DK/NIDDK NIH HHS/United States
- R37 DK031405-31/DK/NIDDK NIH HHS/United States
- S10 RR027270/RR/NCRR NIH HHS/United States
- R01-GM084041/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases