Amyloid-β-induced synapse damage is mediated via cross-linkage of cellular prion proteins - PubMed (original) (raw)
Amyloid-β-induced synapse damage is mediated via cross-linkage of cellular prion proteins
Clive Bate et al. J Biol Chem. 2011.
Abstract
The cellular prion protein (PrP(C)), which is highly expressed at synapses, was identified as a receptor for the amyloid-β (Aβ) oligomers that are associated with dementia in Alzheimer disease. Here, we report that Aβ oligomers secreted by 7PA2 cells caused synapse damage in cultured neurons via a PrP(C)-dependent process. Exogenous PrP(C) added to Prnp knock-out((0/0)) neurons was targeted to synapses and significantly increased Aβ-induced synapse damage. In contrast, the synapse damage induced by a phospholipase A(2)-activating peptide was independent of PrP(C). In Prnp wild-type((+/+)) neurons Aβ oligomers activated synaptic cytoplasmic phospholipase A(2) (cPLA(2)). In these cells, the addition of Aβ oligomers triggered the translocation of cPLA(2) in synapses to cholesterol dense membranes (lipid rafts) where it formed a complex also containing Aβ and PrP(C). In contrast, the addition of Aβ to Prnp((0/0)) neurons did not activate synaptic cPLA(2), which remained in the cytoplasm and was not associated with Aβ. Filtration assays and non-denaturing gels demonstrated that Aβ oligomers cross-link PrP(C). We propose that it is the cross-linkage of PrP(C) by Aβ oligomers that triggers abnormal activation of cPLA(2) and synapse damage. This hypothesis was supported by our observation that monoclonal antibody mediated cross-linkage of PrP(C) also activated synaptic cPLA(2) and caused synapse damage.
Figures
FIGURE 1.
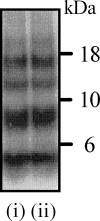
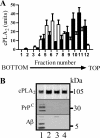
PrPC mediates Aβ-induced synapse damage. A, immunoblot showing Aβ monomers, dimers, trimers, and tetramers in concentrated 7PA2-CM (i) or CHO-CM (ii) separated by non-denaturing PAGE and detected with mAb 6E10. B, the amount of synaptophysin in cortical neurons derived from Prnp(+/+) mice (●) or Prnp(0/0) mice (○) and incubated with Aβ42 for 24 h. Values shown are the mean amount of synaptophysin (units) ± S.D. from triplicate experiments performed five times (n = 15). C, immunoblots showing the amount of synaptophysin (i), synaptobrevin (ii), and β-actin (iii) in Prnp(+/+) cortical neurons incubated with Aβ42 as shown for 24 h. D, the amount of synaptophysin in cortical neurons derived from Prnp(+/+) (●) or Prnp(0/0) mice (○) and incubated with PLAP for 24 h. Values shown are the mean amount of synaptophysin (units) ± S.D. from triplicate experiments performed four times (n = 12).
FIGURE 2.
Aβ accumulates at synaptosomes in the absence of PrPC. Immunoblot showing Aβ monomers, dimers, trimers, and tetramers in synaptosomes isolated from cortical neurons derived from Prnp(+/+) mice (i) or Prnp(0/0) mice (ii) and incubated with 10 ng of Aβ42 for 1 h. Synaptosomes were electrophoresed under non-denaturing conditions, and Aβ was detected with mAb 6E10.
FIGURE 3.
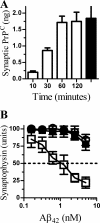
PrPC increases Aβ-induced synapse damage in Prnp(0/0) neurons. A, the amount of PrPC found in synaptosomes derived from Prnp(0/0) neurons incubated with 20 ng of PrPC for the time periods as shown (□) or from Prnp(+/+) synaptosomes (■). Values shown are the mean amount of synaptic PrPC (ng/106 cells) ± S.D. from duplicate experiments performed five times (n = 10). B, the synaptophysin content of Prnp(0/0) neurons pre-treated with control medium (○), 20 ng PrPC (□), or 20 ng Thy-1 (■) and incubated for 24 h with Aβ42. Values shown are the mean amount of synaptophysin (units) ± S.D. from triplicate experiments performed four times (n = 12).
FIGURE 4.
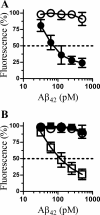
PrPC mediates Aβ-induced inhibition of synaptic vesicle recycling. A, the amount of FM1–43 in cortical neurons derived from Prnp(+/+) (●) or Prnp(0/0) (○) mice, which were incubated with Aβ42 derived from 7PA2-CM, as shown for 24 h. Cells were then pulsed with 1 μg/ml FM1-43 and 1 μ
m
ionomycin for 5 min. Values shown are the mean amounts of FM1-43 (% fluorescence) ± S.D. from triplicate experiments performed four times (n = 12). B, the amount of FM1-43 in cortical neurons derived from Prnp(0/0) mice that had been pre-treated with control medium (○), 20 ng of PrPC (□), or 20 ng of Thy-1 (■) and incubated with Aβ42 as shown for 24 h. Cells were then pulsed with 1 μg/ml FM1-43 and 1 μ
m
ionomycin for 5 min. Values shown are the mean amounts of FM1-43 (% fluorescence) ± S.D. from triplicate experiments performed four times (n = 12).
FIGURE 5.
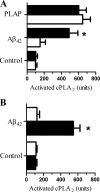
PrPC is required for Aβ-induced activation of cPLA2. A, the amount of activated cPLA2 in synaptosomes isolated from neurons derived from Prnp(+/+) (■) or Prnp(0/0) mice (□), which had been incubated for 1 h with control medium, 2 n
m
Aβ42, or 500 ng/ml PLAP. Values shown are the mean amount of activated cPLA2 (units) ± S.D. from triplicate experiments performed four times (n = 12). An asterisk represents a significant difference between Prnp(+/+) and Prnp(0/0) synaptosomes (p < 0.05). B, the amount of activated cPLA2 in synaptosomes isolated from Prnp(0/0) neurons, which were pre-treated with 20 ng of PrPC (■) or 20 ng of Thy-1 (□), and incubated for 1 h with control medium or 2 n
m
Aβ42. Values shown are the mean amount of activated cPLA2 (units) ± S.D. from triplicate experiments performed four times (n = 12). An asterisk represents a significant difference between treated synaptosomes (p < 0.05).
FIGURE 6.
PrPC is required for the Aβ-induced translocation of cPLA2. A, synaptosomes were isolated from Prnp(+/+) (■) or Prnp(0/0) (□) neurons that had been incubated with 2 n
m
Aβ42 for 1 h. Extracts were prepared and separated on sucrose density gradients and the amount of cPLA2 in each fraction was determined by ELISA. Values shown are the mean amount of cPLA2 (units) ± S.D. from triplicate experiments performed three times (n = 9). B, immunoblot showing the amounts of cPLA2, PrPC, and Aβ precipitated by mAb CH-7 (anti-cPLA2) from Prnp(+/+) synaptosomes incubated with 2 n
m
Aβ42 (lane 1) or CHO-CM (lane 2). Also shown are precipitates from Prnp(0/0) synaptosomes incubated with 2 n
m
Aβ42 (lane 3) and precipitates from Prnp(+/+) synaptosomes incubated with 2 n
m
Aβ42 and homogenized in 0.2% SDS that disperses lipid rafts (lane 4).
FIGURE 7.
Aβ cross-links PrPC. A, synaptosomes derived from Prnp(+/+) neurons were incubated with 2 n
m
Aβ42 or CHO-CM for 1 h. Extracts were separated by non-denaturing gel electrophoresis and probed for PrPC. Immunoblot showing PrPC derived from synaptosomes incubated with Aβ oligomers derived from 7PA2 cells (lane 1) or CHO-CM (lane 2). B, soluble PrPC was incubated with CHO-CM (□) or 7PA2-CM containing 2 n
m
Aβ42 (■) for 1 h and then passed through 50- or 300-kDa filters. The amount of PrPC that passed through each filter was measured. Values shown are the mean amount of PrPC ± S.D. from triplicate experiments performed four times (n = 12).
FIGURE 8.
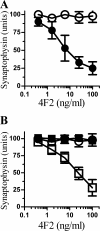
A mAb to PrPC causes synapse damage. A, the amount of synaptophysin in cortical neurons derived from Prnp(+/+) mice (●) or Prnp(0/0) mice (○) and incubated with different concentrations of the PrPC-reactive mAb 4F2 for 24 h. Values shown are the mean amount of synaptophysin (units) ± S.D. from triplicate experiments performed five times (n = 15). B, the synaptophysin content of cortical neurons derived from Prnp(0/0) mice pre-treated with control medium (○), 20 ng of PrPC (□), or 20 ng of Thy-1 (■) and incubated for 24 h with different concentrations of mAb 4F2. Values shown are the mean amount of synaptophysin (units) ± S.D. from triplicate experiments performed three times (n = 9).
FIGURE 9.
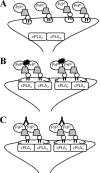
PrPC mediates the activation of cPLA2 and synapse damage induced by Aβ. Shown is a schematic showing the proposed relationship between PrPC, cPLA2, lipid rafts (open clouds), and Aβ oligomers (black ink blots). A, in the absence of Aβ oligomers, cPLA2 and PrPC and are not associated, and there is no activation of cPLA2 or synapse damage. B, in Prnp(+/+) synapses, Aβ oligomers cross-link PrPC resulting in a complex containing Aβ, PrPC, and cPLA2, which leads to the activation of cPLA2 and synapse damage. C, the addition of mAb 4F2 also cross-links PrPC resulting in the association between cPLA2 and PrPC, activation of cPLA2, and synapse damage.
Similar articles
- Monomeric amyloid-β reduced amyloid-β oligomer-induced synapse damage in neuronal cultures.
Bate C, Williams A. Bate C, et al. Neurobiol Dis. 2018 Mar;111:48-58. doi: 10.1016/j.nbd.2017.12.007. Epub 2017 Dec 19. Neurobiol Dis. 2018. PMID: 29272738 - Glimepiride protects neurons against amyloid-β-induced synapse damage.
Osborne C, West E, Nolan W, McHale-Owen H, Williams A, Bate C. Osborne C, et al. Neuropharmacology. 2016 Feb;101:225-36. doi: 10.1016/j.neuropharm.2015.09.030. Epub 2015 Oct 8. Neuropharmacology. 2016. PMID: 26432105 - Clustering of sialylated glycosylphosphatidylinositol anchors mediates PrP-induced activation of cytoplasmic phospholipase A 2 and synapse damage.
Bate C, Williams A. Bate C, et al. Prion. 2012 Sep-Oct;6(4):350-3. doi: 10.4161/pri.21751. Epub 2012 Aug 16. Prion. 2012. PMID: 22895089 Free PMC article. - Cellular prion protein: A co-receptor mediating neuronal cofilin-actin rod formation induced by β-amyloid and proinflammatory cytokines.
Walsh KP, Kuhn TB, Bamburg JR. Walsh KP, et al. Prion. 2014;8(6):375-80. doi: 10.4161/pri.35504. Prion. 2014. PMID: 25426519 Free PMC article. Review. - The pathogenesis of soluble PrP fragments containing Aβ binding sites.
Li B. Li B. Virus Res. 2016 Jan 4;211:194-8. doi: 10.1016/j.virusres.2015.10.023. Epub 2015 Oct 31. Virus Res. 2016. PMID: 26528810 Review.
Cited by
- Molecular Factors Mediating Neural Cell Plasticity Changes in Dementia Brain Diseases.
Kozubski W, Ong K, Waleszczyk W, Zabel M, Dorszewska J. Kozubski W, et al. Neural Plast. 2021 Mar 29;2021:8834645. doi: 10.1155/2021/8834645. eCollection 2021. Neural Plast. 2021. PMID: 33854544 Free PMC article. Review. - Structural details of amyloid β oligomers in complex with human prion protein as revealed by solid-state MAS NMR spectroscopy.
König AS, Rösener NS, Gremer L, Tusche M, Flender D, Reinartz E, Hoyer W, Neudecker P, Willbold D, Heise H. König AS, et al. J Biol Chem. 2021 Jan-Jun;296:100499. doi: 10.1016/j.jbc.2021.100499. Epub 2021 Mar 3. J Biol Chem. 2021. PMID: 33667547 Free PMC article. - On the role of the cellular prion protein in the uptake and signaling of pathological aggregates in neurodegenerative diseases.
Legname G, Scialò C. Legname G, et al. Prion. 2020 Dec;14(1):257-270. doi: 10.1080/19336896.2020.1854034. Prion. 2020. PMID: 33345731 Free PMC article. Review. - Binding between Prion Protein and Aβ Oligomers Contributes to the Pathogenesis of Alzheimer's Disease.
Kong C, Xie H, Gao Z, Shao M, Li H, Shi R, Cai L, Gao S, Sun T, Li C. Kong C, et al. Virol Sin. 2019 Oct;34(5):475-488. doi: 10.1007/s12250-019-00124-1. Epub 2019 May 15. Virol Sin. 2019. PMID: 31093882 Free PMC article. Review. - In vivo induction of membrane damage by β-amyloid peptide oligomers.
Julien C, Tomberlin C, Roberts CM, Akram A, Stein GH, Silverman MA, Link CD. Julien C, et al. Acta Neuropathol Commun. 2018 Nov 29;6(1):131. doi: 10.1186/s40478-018-0634-x. Acta Neuropathol Commun. 2018. PMID: 30497524 Free PMC article.
References
- Selkoe D. J. (2002) Science 298, 789–791 - PubMed
- Tanzi R. E. (2005) Nat. Neurosci. 8, 977–979 - PubMed
- Vassar R., Citron M. (2000) Neuron 27, 419–422 - PubMed
- Vardy E. R., Catto A. J., Hooper N. M. (2005) Trends Mol. Med. 11, 464–472 - PubMed
- Hardy J., Selkoe D. J. (2002) Science 297, 353–356 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials