Predisposition to cancer caused by genetic and functional defects of mammalian Atad5 - PubMed (original) (raw)
. 2011 Aug;7(8):e1002245.
doi: 10.1371/journal.pgen.1002245. Epub 2011 Aug 25.
Nilabja Sikdar, Kyoo-Young Lee, Jessica C Price, Raghunath Chatterjee, Hee-Dong Park, Jennifer Fox, Masamichi Ishiai, Meghan L Rudd, Lana M Pollock, Sarah K Fogoros, Hassan Mohamed, Christin L Hanigan; NISC Comparative Sequencing Program; Suiyuan Zhang, Pedro Cruz, Gabriel Renaud, Nancy F Hansen, Praveen F Cherukuri, Bhavesh Borate, Kirk J McManus, Jan Stoepel, Payal Sipahimalani, Andrew K Godwin, Dennis C Sgroi, Maria J Merino, Gene Elliot, Abdel Elkahloun, Charles Vinson, Minoru Takata, James C Mullikin, Tyra G Wolfsberg, Philip Hieter, Dae-Sik Lim, Kyungjae Myung
Affiliations
- PMID: 21901109
- PMCID: PMC3161924
- DOI: 10.1371/journal.pgen.1002245
Predisposition to cancer caused by genetic and functional defects of mammalian Atad5
Daphne W Bell et al. PLoS Genet. 2011 Aug.
Abstract
ATAD5, the human ortholog of yeast Elg1, plays a role in PCNA deubiquitination. Since PCNA modification is important to regulate DNA damage bypass, ATAD5 may be important for suppression of genomic instability in mammals in vivo. To test this hypothesis, we generated heterozygous (Atad5(+/m)) mice that were haploinsuffficient for Atad5. Atad5(+/m) mice displayed high levels of genomic instability in vivo, and Atad5(+/m) mouse embryonic fibroblasts (MEFs) exhibited molecular defects in PCNA deubiquitination in response to DNA damage, as well as DNA damage hypersensitivity and high levels of genomic instability, apoptosis, and aneuploidy. Importantly, 90% of haploinsufficient Atad5(+/m) mice developed tumors, including sarcomas, carcinomas, and adenocarcinomas, between 11 and 20 months of age. High levels of genomic alterations were evident in tumors that arose in the Atad5(+/m) mice. Consistent with a role for Atad5 in suppressing tumorigenesis, we also identified somatic mutations of ATAD5 in 4.6% of sporadic human endometrial tumors, including two nonsense mutations that resulted in loss of proper ATAD5 function. Taken together, our findings indicate that loss-of-function mutations in mammalian Atad5 are sufficient to cause genomic instability and tumorigenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
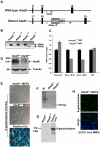
Figure 1. Disruption of the mouse Atad5 gene by insertional mutagenesis.
(A) Schematic diagram of wild type and mutant Atad5 loci. Exons 17–19 are indicated as solid boxes. LacZ and NEO indicate the location of the cassette that disrupted Atad5 gene expression by insertional mutagenesis. Arrows and numbers between arrows indicate the locations of primers used for genotyping and expected sizes of PCR products, respectively. PCR with gray and black primer that can bind wild type locus generates 542 bp PCR products from wild type loci but not in mutant locus due to the inserted LacZ-NEO cassette. In contrast, PCR with gray and another black primer that can bind LacZ sequence generates 900 bp PCR product only from mutant locus. (B) PCR based genotyping of DNAs isolated from mouse embryos at day 3.5 p.c. Wild type and mutant alleles of Atad5 are represented by + and m, respectively. 1st and 2nd lanes in each sample shows PCR products generated from wild type locus (542 bp) and mutant locus (900 bp) explained in the legend A. (C) RT-PCR analysis of Atad5 mRNA levels in wild type and Atad5+/m MEFs. The exons to which each Atad5 primers bind are indicated on the x-axis. (D) Western blot analysis of Atad5 in wild type MEFs and Atad5+/m MEFs. (E) β galactosidase expression in wild type (Atad5+/+; negative control), Atad5+/m MEF cells, and HEK 293T cells transiently transfected with a plasmid expressing β-galactosidase (positive control) were examined by immunostaining with a β-galactosidase antibody. (F) The fused Atad5-LacZ transcript was confirmed by RT-PCR. M stands for molecular marker. A 210 bp RT-PCR product only from Atad5+/m cDNA MEF cells was detected. (G) β galactosidase expression in wild type (Atad5+/+; negative control), Atad5+/m MEF cells, and HEK 293T cells transiently transfected with a plasmid expressing β-galactosidase (positive control) were determined by Western blot analysis. (H) Atad5- β-galactosidase fusion protein was not detected in the Atad5+/m MEF cells even after 12 hours recovery from 0.01% MMS treatment for one hour by immunostaining with a β-galactosidase antibody. Top and bottom panels are images of β-galactosidase antibody staining only and merged with DAPI, respectively.
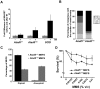
Figure 2. The heterozygous Atad5+/m mutation causes genomic instability in vivo and in MEFs.
(A) Atad5+/m mice exhibited an increase in IR-induced MN-RETs. Wild type (Atad5+/+) and SCID mice were treated with IR and their peripheral blood cells were analyzed in the same manner for comparison. CD71 and propidium iodide (PI) were used to detect RET cells and micronuclei, respectively. Fold induction after IR irradiation in each mouse with a different genotype normalized to MN-RET from unirradiated mice is presented in the graph. Five wild type, five Atad5+/m, and two SCID mice were analyzed. (B) MEFs derived from the Atad5+/m mice produced more chromatid breaks than wild type MEFs in response to MMS treatment. Percentages of metaphases having noted number of breaks are presented as graphs. Noticeable chromatid breaks per cell were quantified for 50 metaphases from both wild type and the Atad5+/m MEFs. (C) MEFs derived from the Atad5+/m mice have a high level of aneuploidy. (D) Survival of wild type and the Atad5+/m MEFs following MMS treatment is displayed as the percentage of viable cells relative to untreated cells. P-values were calculated or each data point as follows, 0.00125% MMS (p = 0.05); 0.0025% MMS (p = 0.002), 0.005% MMS (p = 0.005), 0.0075% MMS (p = 0.09); and 0.01% MMS (p = 0.03), respectively.
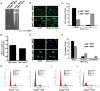
Figure 3. MEFs derived from the Atad5+/m mice are defective in DNA repair, display a hyper-recombination phenotype, and are apoptosis-prone.
(A) Atad5+/m MEFs are defective in DNA repair. Broken DNAs after 0.01% MMS treatment were visualized by pulse field gel electrophoresis. (B, C) Atad5+/m MEFs display high levels of spontaneous Rad51 foci in the nucleus. B and C show, respectively, an actual image and a graphic presentation of the number of Rad51 foci in the nucleus of each cell type. (D) Atad5+/m MEFs display similar rates of sister chromatid exchange rate compared to wild type MEFs. (E, F) Atad5+/m MEFs display high level of γH2AX foci in the nucleus. An actual image (E) and a graphic presentation (F) of the number of cells having different types of γH2AX foci in the nucleus from each cell type are shown. (G, H, I, J) Atad5+/m MEFs undergo apoptosis at the 25th passage. FACS analysis of wild type (G, H) and Atad5+/m MEFs (I, J) from the 4th passage (G, I) and the 25th passage (H, J) were performed based on their DNA contents. Bars in images for Atad5+/+ and Atad5+/m (B and E) indicate 20 µm and 10 µm, respectively.
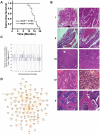
Figure 4. Atad5+/m mice are tumor-prone.
(A) Kaplan-Meier survival graph of wild type (Atad5+/+; n = 20) and the Atad5+/m mice (n = 22). (B) Representative H&E stained sections of tumors that arose in the Atad5+/m mice; (I) Harderian gland adenoma, (II) hemangioma, (III) lung bronchiolar adenoma, (IV) thymoma, and (V) sarcoma from bone marrow. The panel on the right is a two-fold magnification of panel on the left. (C) Frequency of genomic copy number gains and losses among four tumors from the Atad5+/m mice, plotted as a function of genome location with all chromosomes starting from 1pter (left) to X (right). Vertical lines indicate chromosome boundaries. Positive and negative values indicate the number of tumors that showed copy number gains (+) and losses (−), respectively. (D) The pathway activated in two tumors from the Atad5+/m mice analyzed by microarray. Red and green colors represent the activation and inactivation in tumors, respectively.
Figure 5. Somatic mutations of ATAD5 in primary endometrial tumors exhibit functional defects in vitro.
(A) Schematic representation of the ATAD5 protein showing positions of somatic mutations (arrows) relative to the AAA-ATPase domain. Truncating (boxed), missense (black) and synonymous (gray) mutations are distinguished. Amino acids are numbered. (B) The ATAD5-R1414X mutant is associated with reduced protein levels. HEK 293T cells were transfected with constructs expressing wild type (WT) ATAD5 or mutant ATAD5. Soluble fractions, which include cytoplasmic and nucleoplasmic proteins, and chromatin-bound fractions were separated by SDS-PAGE after 48 h. Relative expression of wild type or mutant ATAD5 was determined by Western blotting with anti-FLAG-antibody. Histone H3 and tubulin were used as loading controls for chromatin-bound and soluble fractions respectively. Total RNAs, extracted from cells used in the Western blot analysis, served as templates for RT-PCR. ATAD5 mRNA expression levels determined by quantitative RT-PCR, were normalized to β-actin expression levels and are represented as a relative value compared to that of vector transfectants. (C) The ATAD5-E723X mutant protein does not interact with RFC4. Chromatin-bound fractions from HEK 293T cells transfected with constructs expressing wild type or mutant ATAD5 were immunoprecipitated (IP) with anti-RFC4 antibody or IgG control antibody, and subjected to Western blot analysis.
Similar articles
- Is PCNA unloading the central function of the Elg1/ATAD5 replication factor C-like complex?
Kubota T, Myung K, Donaldson AD. Kubota T, et al. Cell Cycle. 2013 Aug 15;12(16):2570-9. doi: 10.4161/cc.25626. Epub 2013 Jul 10. Cell Cycle. 2013. PMID: 23907118 Free PMC article. Review. - ATAD5 functions as a regulatory platform for Ub-PCNA deubiquitination.
Ryu E, Yoo J, Kang MS, Ha NY, Jang Y, Kim J, Kim Y, Kim BG, Kim S, Myung K, Kang S. Ryu E, et al. Proc Natl Acad Sci U S A. 2024 Aug 20;121(34):e2315759121. doi: 10.1073/pnas.2315759121. Epub 2024 Aug 15. Proc Natl Acad Sci U S A. 2024. PMID: 39145935 - ATAD5 deficiency decreases B cell division and Igh recombination.
Zanotti KJ, Maul RW, Castiblanco DP, Yang W, Choi YJ, Fox JT, Myung K, Saribasak H, Gearhart PJ. Zanotti KJ, et al. J Immunol. 2015 Jan 1;194(1):35-42. doi: 10.4049/jimmunol.1401158. Epub 2014 Nov 17. J Immunol. 2015. PMID: 25404367 Free PMC article. - ATAD5 regulates the lifespan of DNA replication factories by modulating PCNA level on the chromatin.
Lee KY, Fu H, Aladjem MI, Myung K. Lee KY, et al. J Cell Biol. 2013 Jan 7;200(1):31-44. doi: 10.1083/jcb.201206084. Epub 2012 Dec 31. J Cell Biol. 2013. PMID: 23277426 Free PMC article. - PCNA cycling dynamics during DNA replication and repair in mammals.
Kang S, Yoo J, Myung K. Kang S, et al. Trends Genet. 2024 Jun;40(6):526-539. doi: 10.1016/j.tig.2024.02.006. Epub 2024 Mar 13. Trends Genet. 2024. PMID: 38485608 Review.
Cited by
- Ubiquitin and Ubiquitin-Like Proteins Are Essential Regulators of DNA Damage Bypass.
Wilkinson NA, Mnuskin KS, Ashton NW, Woodgate R. Wilkinson NA, et al. Cancers (Basel). 2020 Oct 2;12(10):2848. doi: 10.3390/cancers12102848. Cancers (Basel). 2020. PMID: 33023096 Free PMC article. Review. - The exomes of the NCI-60 panel: a genomic resource for cancer biology and systems pharmacology.
Abaan OD, Polley EC, Davis SR, Zhu YJ, Bilke S, Walker RL, Pineda M, Gindin Y, Jiang Y, Reinhold WC, Holbeck SL, Simon RM, Doroshow JH, Pommier Y, Meltzer PS. Abaan OD, et al. Cancer Res. 2013 Jul 15;73(14):4372-82. doi: 10.1158/0008-5472.CAN-12-3342. Epub 2013 Jul 15. Cancer Res. 2013. PMID: 23856246 Free PMC article. - A role for the yeast PCNA unloader Elg1 in eliciting the DNA damage checkpoint.
Sau S, Kupiec M. Sau S, et al. Curr Genet. 2020 Feb;66(1):79-84. doi: 10.1007/s00294-019-01020-7. Epub 2019 Jul 22. Curr Genet. 2020. PMID: 31332476 Review. - The Role of Co-Deleted Genes in Neurofibromatosis Type 1 Microdeletions: An Evolutive Approach.
Brussa Reis L, Turchetto-Zolet AC, Fonini M, Ashton-Prolla P, Rosset C. Brussa Reis L, et al. Genes (Basel). 2019 Oct 24;10(11):839. doi: 10.3390/genes10110839. Genes (Basel). 2019. PMID: 31652930 Free PMC article. - Genome-wide profiling of genetic synthetic lethality identifies CDK12 as a novel determinant of PARP1/2 inhibitor sensitivity.
Bajrami I, Frankum JR, Konde A, Miller RE, Rehman FL, Brough R, Campbell J, Sims D, Rafiq R, Hooper S, Chen L, Kozarewa I, Assiotis I, Fenwick K, Natrajan R, Lord CJ, Ashworth A. Bajrami I, et al. Cancer Res. 2014 Jan 1;74(1):287-97. doi: 10.1158/0008-5472.CAN-13-2541. Epub 2013 Nov 15. Cancer Res. 2014. PMID: 24240700 Free PMC article.
References
- Foijer F. CINister thoughts. Biochem Soc Trans. 2010;38:1715–1721. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA140323/CA/NCI NIH HHS/United States
- MOP-38096/CAPMC/ CIHR/Canada
- R01-1CA112021-01/CA/NCI NIH HHS/United States
- R01 CA112021/CA/NCI NIH HHS/United States
- U01 CA113916/CA/NCI NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous