IKBKE protein activates Akt independent of phosphatidylinositol 3-kinase/PDK1/mTORC2 and the pleckstrin homology domain to sustain malignant transformation - PubMed (original) (raw)
IKBKE protein activates Akt independent of phosphatidylinositol 3-kinase/PDK1/mTORC2 and the pleckstrin homology domain to sustain malignant transformation
Jian-Ping Guo et al. J Biol Chem. 2011.
Retraction in
- IKBKE protein activates Akt independent of phosphatidylinositol 3-kinase/PDK1/mTORC2 and the pleckstrin homology domain to sustain malignant transformation.
Guo JP, Coppola D, Cheng JQ. Guo JP, et al. J Biol Chem. 2016 Oct 21;291(43):22853. doi: 10.1074/jbc.A111.287433. J Biol Chem. 2016. PMID: 27825091 Free PMC article. No abstract available.
Abstract
Serine/threonine kinase Akt regulates key cellular processes such as cell growth, proliferation, and survival. Activation of Akt by mitogenic factor depends on phosphatidylinositol 3-kinase (PI3K). Here, we report that IKBKE (also known as IKKε and IKKi) activates Akt through a PI3K-independent pathway. IKBKE directly phosphorylates Akt-Thr308 and Ser473 independent of the pleckstrin homology (PH) domain. IKBKE activation of Akt was not affected by inhibition of PI3K, knockdown of PDK1 or mTORC2 complex. Further, this activation could be inhibited by Akt inhibitors MK-2206 and GSK690693 but not the compounds (perifosine and triciribine) targeting the PH domain of Akt. Expression of IKBKE largely correlates with activation of Akt in breast cancer. Moreover, inhibition of Akt suppresses IKBKE oncogenic transformation. These findings indicate that IKBKE is an Akt-Thr308 and -Ser473 kinase and directly activates Akt independent of PI3K, PDK1, and mTORC2 as well as PH domain. Our data also suggest that Akt inhibitors targeting the PH domain have no effect on the tumors in which hyperactive Akt resulted from elevated IKBKE.
Figures
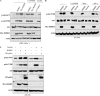
FIGURE 1.
IKBKE activates Akt. A, HeLa cells were transfected with myc-tagged different forms of IKBKE. Following 48 h incubation and overnight starvation, Akt was immunoprecipitated with anti-Akt antibody and subjected to in vitro kinase assay using histone H2B as substrate (top). Panels 2–7 are Western blots detected with indicated antibodies. B and C, HeLa and H1299 cells were transfected with Myc-tagged IKBKEs and probed as A. D and E, H157 cells were treated with siRNA of IKBKE for 72 h and then subjected to in vitro Akt kinase (D) and immunoblotting analysis (E). F, _Ikbke_-null MEFs were transfected with IKBKE and control vectors and immunoblotted with indicated antibodies. Each experiment was repeated three times. Phospho-Akt was quantified relative to total Akt using Image-Quant software. Basal levels of Akt activity and pAkt (e.g. left lane of panels A–F) were referred to 1.0, which was used to calculate the effect of IKBKE on Akt activation.
FIGURE 2.
IKBKE directly phosphorylates Akt-Thr308 and -Ser473 in vitro and in vivo. A, in vitro IKBKE kinase assay by incubating recombinant IKBKE and recombinant Akt1 and Akt2 proteins, which were used as substrates, in a kinase buffer containing [32P]ATP (top). Bottom panel is Coomassie Blue staining of Akt proteins. B, immunoblotting analysis of the in vitro IKBKE kinase reaction described as A with indicated antibodies. C, HeLa cells were transfected with HA-K179M-Akt (dominant-negative Akt) and -K179M-Akt-T308A/S473A and were immunoprecipitated with anti-HA antibody. The immunoprecipitates were subjected to in vitro IKBKE kinase assay (top). Bottom panel is an immunoblot showing expression of transfected Akt. D, in vivo labeling. HeLa cells were transfected with HA-K179M-Akt and -K179M-Akt-T308A/S473A together with and without myr-IKBKE. After [32P]orthophosphate labeling, Akt was immunoprecipitated with anti-HA antibody, separated in SDS-PAGE and exposed (top). Expression of transfected plasmids was shown in panels 2 and 3. E, HeLa cells were transfected with HA-K179M-Akt, -K179M-Akt-T308A, -K179M-Akt-S473A, and -K179M-Akt-T308A/S473A together with and without myr-IKBKE. After immunoprecipitated with anti-HA antibody, the immunoprecipitates were subjected to Western blot analysis with indicated antibodies (panels 1–3). Bottom panel shows expression of myr-IKBKE. F, HeLa cells were transfected with and without myr-IKBKE and immunoprecipitated with Akt member-specific antibodies. The immunoprecipitates were immunoblotted with indicated antibodies (Note: antibody against Akt is a pan-Akt antibody). Expression of transfected myr-IKBKE was shown in the bottom panel. Levels of pAkt in the cell without transfection of myr-IKBKE were referred to 1.0, which was used to calculate IKBKE-induced pAkt1, pAkt2, and pAkt3. Phospho-Akt in panel B was quantified as described in Fig. 1.
FIGURE 3.
IKBKE activates Akt independent of PI3K. A, _Ikbke_-knock-out MEFs were transfected with wild-type and constitutively active IKBKE, treated with PI3K inhibitor LY294002 and then immunoblotted with indicated antibodies. The cells stimulated with insulin and treated with LY294002 were used as control (right lanes). B, Western blot analysis of H1299 cells, which were transfected with wild-type IKBKE and treated insulin and inhibitors of PI3K (LY294002, 10 μ
m
and Wortmannin 1 μ
m
) and Akt (API-2, 10 μ
m
), with indicated antibodies. C, H1299 cells were transfected with DN-p85α and IKBKE and then were treated with/without insulin. pAkt and expression of transfected plasmids were detected with Western blot.
FIGURE 4.
PH domain of Akt, PDK1, and mTORC2 are not required for IKBKE activation of Akt; Akt inhibitors targeting the PH domain of Akt did not inhibit IKBKE-activated Akt. A, H1299 cells were transfected PH domain truncated Akt together with three different forms of IKBKE and immunoblotted with indicated antibodies. The cells treated with insulin were used as control (right lane). B, IKBKE-transfected or insulin-simulated H1299 cells were treated with indicated Akt inhibitors (e.g. perifosine 5 μ
m
, MK2206 10 μ
m
, and GSK690693 10 μ
m
) and probed as A. C, following transfection of _PDK1_-null and parental HCT116 cells with HA-Akt, wild-type and constitutively active IKBKE, Western blot analysis was performed. D, H1299 cells were transfected/treated with siRNAs of Rictor and mTOR or rapamycin (100 n
m
for 24 h) together with and without IKBKE and then immunoblotted with indicated antibodies. Note: Rictor and mTOR were knocked down at ∼85 and ∼70%, respectively. Phospho-Akt in the cells transfected with IKBKE and control siRNA (second left lane) was referred to 1.0, which was served as basal level for calculation of pAkt in the rest lanes.
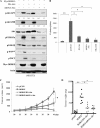
FIGURE 5.
Elevated IKBKE correlates with hyperactive Akt in breast cancer. Immunoblotting (A) and immunohistochemical staining (B; arrows, tumor and arrowheads, stromal tissue) of representative primary breast tumors with antibodies against pAkt, IKBKE, Akt, and actin. C, Chi-square test analysis of expression of IKBKE and pAkt in 98 breast cancer specimens examined. The elevated IKBKE significantly correlates with hyperactive Akt (p = 0.035).
FIGURE 6.
Akt mediates IKBKE oncogenic activity. A, NIH3T3 cells were transfected with constitutively active IKBKE alone or together with DN-Akt or shRNA/Akt and immunoblotted with indicated antibodies. Phospho-Akt was quantified as described in Fig. 1. B, anchorage-independent growth of the transfectants was assessed for 21 days. Inhibition of Akt significantly reduces IKBKE-induced colony formation. Error bars depict S.D. for three independent experiments. C and D, 1 × 106 cells from each transfectant were subcutaneously injected to nude mouse (8 mice/transfectant). Tumor growth (C) and weight (D) were evaluated. Asterisks indicate p < 0.05.
Similar articles
- IkappaB kinase epsilon and TANK-binding kinase 1 activate AKT by direct phosphorylation.
Xie X, Zhang D, Zhao B, Lu MK, You M, Condorelli G, Wang CY, Guan KL. Xie X, et al. Proc Natl Acad Sci U S A. 2011 Apr 19;108(16):6474-9. doi: 10.1073/pnas.1016132108. Epub 2011 Apr 4. Proc Natl Acad Sci U S A. 2011. PMID: 21464307 Free PMC article. - 3-Phosphoinositide-dependent kinase 1 potentiates upstream lesions on the phosphatidylinositol 3-kinase pathway in breast carcinoma.
Maurer M, Su T, Saal LH, Koujak S, Hopkins BD, Barkley CR, Wu J, Nandula S, Dutta B, Xie Y, Chin YR, Kim DI, Ferris JS, Gruvberger-Saal SK, Laakso M, Wang X, Memeo L, Rojtman A, Matos T, Yu JS, Cordon-Cardo C, Isola J, Terry MB, Toker A, Mills GB, Zhao JJ, Murty VV, Hibshoosh H, Parsons R. Maurer M, et al. Cancer Res. 2009 Aug 1;69(15):6299-306. doi: 10.1158/0008-5472.CAN-09-0820. Epub 2009 Jul 14. Cancer Res. 2009. PMID: 19602588 Free PMC article. - PI3 kinase directly phosphorylates Akt1/2 at Ser473/474 in the insulin signal transduction pathway.
Tsuchiya A, Kanno T, Nishizaki T. Tsuchiya A, et al. J Endocrinol. 2013 Nov 28;220(1):49-59. doi: 10.1530/JOE-13-0172. Print 2014 Jan. J Endocrinol. 2013. PMID: 24169049 Free PMC article. - Crosstalk between STAT5 activation and PI3K/AKT functions in normal and transformed mammary epithelial cells.
Rädler PD, Wehde BL, Wagner KU. Rädler PD, et al. Mol Cell Endocrinol. 2017 Aug 15;451:31-39. doi: 10.1016/j.mce.2017.04.025. Epub 2017 May 8. Mol Cell Endocrinol. 2017. PMID: 28495456 Free PMC article. Review. - Phosphorylation of Akt at the C-terminal tail triggers Akt activation.
Liu P, Wang Z, Wei W. Liu P, et al. Cell Cycle. 2014;13(14):2162-4. doi: 10.4161/cc.29584. Epub 2014 Jun 16. Cell Cycle. 2014. PMID: 24933731 Free PMC article. Review.
Cited by
- Inactivation of the enzyme GSK3α by the kinase IKKi promotes AKT-mTOR signaling pathway that mediates interleukin-1-induced Th17 cell maintenance.
Gulen MF, Bulek K, Xiao H, Yu M, Gao J, Sun L, Beurel E, Kaidanovich-Beilin O, Fox PL, DiCorleto PE, Wang JA, Qin J, Wald DN, Woodgett JR, Jope RS, Carman J, Dongre A, Li X. Gulen MF, et al. Immunity. 2012 Nov 16;37(5):800-12. doi: 10.1016/j.immuni.2012.08.019. Epub 2012 Nov 8. Immunity. 2012. PMID: 23142783 Free PMC article. - TBK1 mediates critical effects of measles virus nucleocapsid protein (MVNP) on pagetic osteoclast formation.
Sun Q, Sammut B, Wang FM, Kurihara N, Windle JJ, Roodman GD, Galson DL. Sun Q, et al. J Bone Miner Res. 2014 Jan;29(1):90-102. doi: 10.1002/jbmr.2026. J Bone Miner Res. 2014. PMID: 23794264 Free PMC article. - Elevated expression of TANK-binding kinase 1 enhances tamoxifen resistance in breast cancer.
Wei C, Cao Y, Yang X, Zheng Z, Guan K, Wang Q, Tai Y, Zhang Y, Ma S, Cao Y, Ge X, Xu C, Li J, Yan H, Ling Y, Song T, Zhu L, Zhang B, Xu Q, Hu C, Bian XW, He X, Zhong H. Wei C, et al. Proc Natl Acad Sci U S A. 2014 Feb 4;111(5):E601-10. doi: 10.1073/pnas.1316255111. Epub 2014 Jan 21. Proc Natl Acad Sci U S A. 2014. PMID: 24449872 Free PMC article. - Deregulated expression of TANK in glioblastomas triggers pro-tumorigenic ERK1/2 and AKT signaling pathways.
Stellzig J, Chariot A, Shostak K, Ismail Göktuna S, Renner F, Acker T, Pagenstecher A, Schmitz ML. Stellzig J, et al. Oncogenesis. 2013 Nov 11;2(11):e79. doi: 10.1038/oncsis.2013.42. Oncogenesis. 2013. PMID: 24217713 Free PMC article. - Detection of IKKε by immunohistochemistry in primary breast cancer: association with EGFR expression and absence of lymph node metastasis.
Williams V, Grosset AA, Zamorano Cuervo N, St-Pierre Y, Sylvestre MP, Gaboury L, Grandvaux N. Williams V, et al. BMC Cancer. 2017 May 22;17(1):356. doi: 10.1186/s12885-017-3321-6. BMC Cancer. 2017. PMID: 28532474 Free PMC article.
References
- Ozes O. N., Mayo L. D., Gustin J. A., Pfeffer S. R., Pfeffer L. M., Donner D. B. (1999) Nature 401, 82–85 - PubMed
- Brunet A., Bonni A., Zigmond M. J., Lin M. Z., Juo P., Hu L. S., Anderson M. J., Arden K. C., Blenis J., Greenberg M. E. (1999) Cell 96, 857–868 - PubMed
- Inoki K., Li Y., Zhu T., Wu J., Guan K. L. (2002) Nat. Cell Biol. 4, 648–657 - PubMed
- Shin I., Yakes F. M., Rojo F., Shin N. Y., Bakin A. V., Baselga J., Arteaga C. L. (2002) Nat. Med. 8, 1145–1152 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous