Integrins in tumor angiogenesis and lymphangiogenesis - PubMed (original) (raw)
Integrins in tumor angiogenesis and lymphangiogenesis
Philippe Foubert et al. Methods Mol Biol. 2012.
Abstract
Angiogenesis, the formation of new blood vessel, plays an important role for the growth and metastasis of malignant tumors. The recent identification of specific growth factors for lymphatic vessels and of new lymphatic-specific markers provided evidence for an active role of the lymphatic system during the tumor growth and metastasis processes. Tumor lymphangiogenesis has been shown to play a role in promoting tumor growth and metastasis of tumor cells to distant sites. Integrins play keys roles in the regulation of angiogenesis and lymphangiogenesis during normal development and several diseases. Indeed, integrins control vascular and lymphatic endothelial cell adhesion, migration, and survival. Importantly, integrin inhibitors can block angiogenesis and lymphangiogenesis. In this chapter, we will highlight the role of integrins during angiogenesis and lymphangiogenesis as well as the function of individual integrins during vascular development, postnatal angiogenesis, and lymphangiogenesis. We discuss the role of integrins as potential therapeutic targets for the control of tumor angiogenesis, lymphangiogenesis, and metastatic spread in the treatment of cancer. We also describe methods to analyze expression and function of integrins during angiogenesis and lymphangiogenesis.
Figures
Figure 1
The integrin family of adhesion of adhesion receptors and their ligands. There are 18 α and 8 β subunits which assemble to form 24 different heterodimers. Heterodimer composition confers ligand specificity. The main ligands for integrins in the extracellular space are extracellular matrix proteins, such as laminin, collagen, vitronectin and fibronectin. Moreover, integrins can also bind cellular counter-receptors (VCAM-1 or ICAM-1) and soluble molecules (fibrinogen).
Figure 2
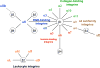
Role of integrins in tumor angiogenesis and lymphangiogenesis. The tumor microenvironment activates or upregulates expression of integrins such as α1β1, α2β1, α4β1, α5β1 and αvβ3 on blood vessels and α1β1, α2β1, α4β1 and α9β1 on lymphatic vessels. Then, these integrins promote endothelial and lymphatic cells migration and survival during invasion of tumor tissue. Angiogenesis and lymphangiogenesis promote metastasis to local and distant organ such as lung.
Similar articles
- Methods to study lymphatic vessel integrins.
Garmy-Susini B, Makale M, Fuster M, Varner JA. Garmy-Susini B, et al. Methods Enzymol. 2007;426:415-38. doi: 10.1016/S0076-6879(07)26018-5. Methods Enzymol. 2007. PMID: 17697894 Review. - Roles of integrins in tumor angiogenesis and lymphangiogenesis.
Garmy-Susini B, Varner JA. Garmy-Susini B, et al. Lymphat Res Biol. 2008;6(3-4):155-63. doi: 10.1089/lrb.2008.1011. Lymphat Res Biol. 2008. PMID: 19093788 Free PMC article. Review. - Integrins in angiogenesis and lymphangiogenesis.
Avraamides CJ, Garmy-Susini B, Varner JA. Avraamides CJ, et al. Nat Rev Cancer. 2008 Aug;8(8):604-17. doi: 10.1038/nrc2353. Epub 2008 May 22. Nat Rev Cancer. 2008. PMID: 18497750 Free PMC article. Review. - Modeling Proteolytically Driven Tumor Lymphangiogenesis.
Lolas G, Jensen L, Bourantas GC, Tsikourkitoudi V, Syrigos K. Lolas G, et al. Adv Exp Med Biol. 2016;936:107-136. doi: 10.1007/978-3-319-42023-3_6. Adv Exp Med Biol. 2016. PMID: 27739045 - Endothelial cell integrins and COX-2: mediators and therapeutic targets of tumor angiogenesis.
Rüegg C, Dormond O, Mariotti A. Rüegg C, et al. Biochim Biophys Acta. 2004 Mar 4;1654(1):51-67. doi: 10.1016/j.bbcan.2003.09.003. Biochim Biophys Acta. 2004. PMID: 14984767 Review.
Cited by
- ACTN1 interacts with ITGA5 to promote cell proliferation, invasion and epithelial-mesenchymal transformation in head and neck squamous cell carcinoma.
Wang R, Gao Y, Zhang H. Wang R, et al. Iran J Basic Med Sci. 2023 Feb;26(2):200-207. doi: 10.22038/IJBMS.2022.67056.14703. Iran J Basic Med Sci. 2023. PMID: 36742137 Free PMC article. - Molecular Modeling Insights into the Structure and Behavior of Integrins: A Review.
Tvaroška I, Kozmon S, Kóňa J. Tvaroška I, et al. Cells. 2023 Jan 14;12(2):324. doi: 10.3390/cells12020324. Cells. 2023. PMID: 36672259 Free PMC article. Review. - Protein corona: Friend or foe? Co-opting serum proteins for nanoparticle delivery.
Kim W, Ly NK, He Y, Li Y, Yuan Z, Yeo Y. Kim W, et al. Adv Drug Deliv Rev. 2023 Jan;192:114635. doi: 10.1016/j.addr.2022.114635. Epub 2022 Nov 26. Adv Drug Deliv Rev. 2023. PMID: 36503885 Free PMC article. Review. - ITGA5 Promotes Tumor Progression through the Activation of the FAK/AKT Signaling Pathway in Human Gastric Cancer.
Wang JF, Chen YY, Zhang SW, Zhao K, Qiu Y, Wang Y, Wang JC, Yu Z, Li BP, Wang Z, Chen JQ. Wang JF, et al. Oxid Med Cell Longev. 2022 Sep 24;2022:8611306. doi: 10.1155/2022/8611306. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36193075 Free PMC article. - Methyl Gallate Suppresses Tumor Development by Increasing Activation of Caspase3 and Disrupting Tumor Angiogenesis in Melanoma.
Park JK, Yoo MJ, Jang H, Park SY, Choi J, Seol JW. Park JK, et al. Evid Based Complement Alternat Med. 2022 Sep 6;2022:6295910. doi: 10.1155/2022/6295910. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 36110191 Free PMC article.
References
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–6. - PubMed
- Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001;49:507–21. - PubMed
- Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–53. - PubMed
- He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, Alitalo K. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst. 2002;94:819–25. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources