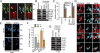Ascl1a/Dkk/beta-catenin signaling pathway is necessary and glycogen synthase kinase-3beta inhibition is sufficient for zebrafish retina regeneration - PubMed (original) (raw)
Ascl1a/Dkk/beta-catenin signaling pathway is necessary and glycogen synthase kinase-3beta inhibition is sufficient for zebrafish retina regeneration
Rajesh Ramachandran et al. Proc Natl Acad Sci U S A. 2011.
Abstract
Key to successful retina regeneration in zebrafish are Müller glia (MG) that respond to retinal injury by dedifferentiating into a cycling population of retinal progenitors. Although recent studies have identified several genes involved in retina regeneration, the signaling mechanisms underlying injury-dependent MG proliferation have remained elusive. Here we report that canonical Wnt signaling controls the proliferation of MG-derived retinal progenitors. We found that injury-dependent induction of Ascl1a suppressed expression of the Wnt signaling inhibitor, Dkk, and induced expression of the Wnt ligand, Wnt4a. Genetic and pharmacological inhibition of Wnt signaling suppressed injury-dependent proliferation of MG-derived progenitors. Remarkably, in the uninjured retina, glycogen synthase kinase-3β (GSK-3β) inhibition was sufficient to stimulate MG dedifferentiation and the formation of multipotent retinal progenitors that were capable of differentiating into all major retinal cell types. Importantly, Ascl1a expression was found to contribute to the multipotential character of these progenitors. Our data suggest that Wnt signaling and GSK-3β inhibition, in particular, are crucial for successful retina regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Ascl1a inhibits the expression of dkk genes during retina regeneration. (A and B) Injury-dependent regulation of Wnt signaling component mRNAs. (C) Double in situ hybridization shows mutually exclusive ascl1a and dkk1b gene expression. (D) ascl1a and dkk1b expression in FACS-purified MG and non-MG from injured retinas. Values are relative to uninjured retina. *P < 0.009. (E and F) Ascl1a knockdown prevents injury-dependent dkk gene suppression. (F) Quantification of E by qPCR. Values are relative to uninjured retina. *P < 0.0001. (G) In situ hybridization showing Ascl1a knockdown relieves injury-dependent dkk suppression. Boxed region in low-magnification image is shown in higher magnification in the row below. Arrows point to ascl1a+/dkk1b+ cells. White dots identify autofluorescence in ONL. (H and I) Injection of zebrafish embryos with dkk1b:gfp-luciferase reporter and increasing amounts of ascl1a mRNA (H) or _ascl1a_-targeting MO (I). *P < 0.005. (J) Lin-28 knockdown differentially affects injury-dependent dkk gene suppression. (K and L) Dkk1b overexpression inhibits cell proliferation at 4 dpi. *P < 0.003. (Scale bars, 10 μm.) ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer.
Fig. 2.
Ascl1a regulates wnt4a expression via a Lin-28 independent pathway. (A) Time course of injury-dependent gene expression. (B and C) MO-mediated Ascl1a knockdown suppresses wnt4a gene induction at 2 dpi. *P < 0.0005. (D) wnt4a expression in MG and non-MG after retinal injury relative to uninjured control retina. *P < 0.0007. (E) ascl1a and wnt4a mRNAs are coexpressed in BrdU+ MG-derived progenitors at 4 dpi. (Scale bar, 10 μm.) (F) Lin-28 knockdown does not suppress injury-dependent wnt4a or fzd2 induction. Abbreviations are as in Fig. 1.
Fig. 3.
β-Catenin accumulation in MG-derived progenitors is necessary for retina regeneration. (A and B) Injury-induced β-catenin expression at 4 dpi is blocked by Dkk1b overexpression in hs:Dkk1GFP fish. (C) Injury-dependent induction of GFP expression in the β-catenin reporter fish, TOPdGFP. (D) Accumulation of β-catenin in GFP+ MG-derived proliferating progenitors of 1016 tuba1a:gfp fish at 4 dpi. (Upper) Standard immunohistochemical protocol. (Lower) Epitope retrieval protocol. (E and F) Pyrvinium and XAV939 inhibit the generation of GFP+, β-catenin+, and BrdU+ MG-derived progenitors at 4 dpi in 1016 tuba1a:gfp fish. White dot identifies an area of autofluorescence that results from long exposures to detect fluorescent signals. *P < 0.001. (Scale bars, 10 μm.) Abbreviations are as in Fig. 1.
Fig. 4.
β-Catenin stabilization stimulates MG dedifferentiation and the generation of multiple retinal cell types in the uninjured retina. (A) Intravitreal injection of GSK-3β inhibitor induces GFP, β-catenin, and cell proliferation in the uninjured retina of 1016 tuba1a:gfp transgenic fish. (B and C) Expression of regeneration-associated genes in uninjured retinas treated with the GSK-3β inhibitor. *P < 0.0001. (D) GSK-3β inhibitor-induced retinal progenitors proliferate and generate all major retinal cell types. (E and F) Effect of Ascl1a knockdown on MG proliferation in the GSK-3β inhibitor-treated retina. *P < 0.002. (G) Effect of Ascl1a knockdown on genes associated with MG dedifferentiation in the GSK-3β inhibitor-treated retina. [Scale bars, 10 μm (A and D); 20 μm (E).] Abbreviations are as in Fig. 1.
Similar articles
- HB-EGF is necessary and sufficient for Müller glia dedifferentiation and retina regeneration.
Wan J, Ramachandran R, Goldman D. Wan J, et al. Dev Cell. 2012 Feb 14;22(2):334-47. doi: 10.1016/j.devcel.2011.11.020. Dev Cell. 2012. PMID: 22340497 Free PMC article. - β-catenin/Wnt signaling controls progenitor fate in the developing and regenerating zebrafish retina.
Meyers JR, Hu L, Moses A, Kaboli K, Papandrea A, Raymond PA. Meyers JR, et al. Neural Dev. 2012 Aug 24;7:30. doi: 10.1186/1749-8104-7-30. Neural Dev. 2012. PMID: 22920725 Free PMC article. - Injury-dependent Müller glia and ganglion cell reprogramming during tissue regeneration requires Apobec2a and Apobec2b.
Powell C, Elsaeidi F, Goldman D. Powell C, et al. J Neurosci. 2012 Jan 18;32(3):1096-109. doi: 10.1523/JNEUROSCI.5603-11.2012. J Neurosci. 2012. PMID: 22262907 Free PMC article. - [Wnt signaling and glucocorticoid-induced osteoporosis].
Ohnaka K. Ohnaka K. Clin Calcium. 2006 Nov;16(11):1812-6. Clin Calcium. 2006. PMID: 17079847 Review. Japanese.
Cited by
- Insm1a-mediated gene repression is essential for the formation and differentiation of Müller glia-derived progenitors in the injured retina.
Ramachandran R, Zhao XF, Goldman D. Ramachandran R, et al. Nat Cell Biol. 2012 Oct;14(10):1013-23. doi: 10.1038/ncb2586. Epub 2012 Sep 23. Nat Cell Biol. 2012. PMID: 23000964 Free PMC article. - Sphingosine-1-phosphate signaling regulates the ability of Müller glia to become neurogenic, proliferating progenitor-like cells.
Taylor O, DeGroff N, El-Hodiri H, Gao C, Fischer AJ. Taylor O, et al. bioRxiv [Preprint]. 2025 Jan 24:2024.08.06.606815. doi: 10.1101/2024.08.06.606815. bioRxiv. 2025. PMID: 39149287 Free PMC article. Updated. Preprint. - Retinal regeneration in adult zebrafish requires regulation of TGFβ signaling.
Lenkowski JR, Qin Z, Sifuentes CJ, Thummel R, Soto CM, Moens CB, Raymond PA. Lenkowski JR, et al. Glia. 2013 Oct;61(10):1687-97. doi: 10.1002/glia.22549. Epub 2013 Aug 5. Glia. 2013. PMID: 23918319 Free PMC article. - Critical Examination of Müller Glia-Derived in vivo Neurogenesis in the Mouse Retina.
Xie Y, Chen B. Xie Y, et al. Front Cell Dev Biol. 2022 Mar 31;10:830382. doi: 10.3389/fcell.2022.830382. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35433694 Free PMC article. Review. - Evidence of regional specializations in regenerated zebrafish retina.
Stenkamp DL, Viall DD, Mitchell DM. Stenkamp DL, et al. Exp Eye Res. 2021 Nov;212:108789. doi: 10.1016/j.exer.2021.108789. Epub 2021 Oct 13. Exp Eye Res. 2021. PMID: 34653519 Free PMC article. Review.
References
- Otteson DC, Hitchcock PF. Stem cells in the teleost retina: Persistent neurogenesis and injury-induced regeneration. Vision Res. 2003;43:927–936. - PubMed
- Mensinger AF, Powers MK. Visual function in regenerating teleost retina following cytotoxic lesioning. Vis Neurosci. 1999;16:241–251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases