Necdin protects embryonic motoneurons from programmed cell death - PubMed (original) (raw)
Necdin protects embryonic motoneurons from programmed cell death
Julianne Aebischer et al. PLoS One. 2011.
Abstract
NECDIN belongs to the type II Melanoma Associated Antigen Gene Expression gene family and is located in the Prader-Willi Syndrome (PWS) critical region. Necdin-deficient mice develop symptoms of PWS, including a sensory and motor deficit. However, the mechanisms underlying the motor deficit remain elusive. Here, we show that the genetic ablation of Necdin, whose expression is restricted to post-mitotic neurons in the spinal cord during development, leads to a loss of 31% of specified motoneurons. The increased neuronal loss occurs during the period of naturally-occurring cell death and is not confined to specific pools of motoneurons. To better understand the role of Necdin during the period of programmed cell death of motoneurons we used embryonic spinal cord explants and primary motoneuron cultures from Necdin-deficient mice. Interestingly, while Necdin-deficient motoneurons present the same survival response to neurotrophic factors, we demonstrate that deletion of Necdin leads to an increased susceptibility of motoneurons to neurotrophic factor deprivation. We show that by neutralizing TNFα this increased susceptibility of Necdin-deficient motoneurons to trophic factor deprivation can be reduced to the normal level. We propose that Necdin is implicated through the TNF-receptor 1 pathway in the developmental death of motoneurons.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
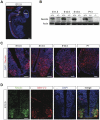
Figure 1. Necdin is expressed in motoneurons during embryonic development and postnatal stage.
(A) Sagital section of whole E12.5 mouse embryo immunostained with a Necdin specific antibody (in red). Section is stained with DAPI (in blue). sc, spinal cord; pt, pyramidal tract; cb, cerebellum; ot, optic tract and soa, supraoptic area. (B) Western blot analysis of Necdin levels in the ventral horn of the lumbar spinal cord at indicated stages. Actin served as a loading control. In absence of the paternal copy of the Necdin gene (the maternal copy is silent due to imprinting mechanism) or in homozygous animals deleted for both copies, Necdin expression is not detected. P, Post-natal day. (C) Immunohistochemical analysis of Necdin expression (in red) in the spinal cord at different stages. Transverse sections were counter-stained with DAPI (in blue). (D) Necdin expression (in green) is mainly restricted to motoneurons, as identified by Islet-1/-2 immunostaining (in red). Later (C), its expression is extended to other cells (E.13.5, E16.5 and P1). Scale bar: A: 500 µm and C, D: 100 µm.
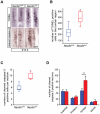
Figure 2. Loss of lumbar specified motoneurons during embryonic development.
Transverse sections of E11.5 (A) and E17.5 (C) lumbar spinal cords were immunolabeled with anti-Islet-1/-2 antibodies. (A) and (B) At E11.5, when pools of post-mitotic motoneurons are generated, no significant difference in the number of Islet-1/-2 positive cells/section is detected between the ventral horn spinal cords of mutant (Necdin+/−) and control (Necdin+/+) mice. (C) and (D) At E17.5, when motoneurons are specified, a significant difference in the number of Islet-1/-2 positive cells/section is observed between both genotypes, with a loss of 31% of cells in _Necdin_-deficient mice (Necdin+/−). Scale bar, 50 µm.
Figure 3. Increased apoptotic motoneuron cell death in the lumbar regions of mutant embryos compared to wildtypes.
A wave of motoneuron developmental cell death is known to occur normally in the lumbar region between the embryonic stages E11.5 and E14.5. (A) Illustration of TUNEL and activated caspase-3 labeling on whole mount spinal cord, focused on the lumbar region at E13.5. (B) Quantification of TUNEL positive cells in the lumbar whole mount spinal cord reveals a significant increase of TUNEL positive cells in mutant (Necdin+/−)(n = 5) compared to wildtype (Necdin+/+) embryos (n = 5). (C) Quantification of activated caspase-3 positive cells on serial transverse sections of the lumbar spinal cord at E13.5 show a significant increase of activated-Casapse-3 positive cells/section in mutant (Necdin+/−) mice (n = 5) compared with the wildtype (Necdin+/+) embryos (n = 5). (D) Quantification of activated caspase-3 positive cells at different levels (brachial, thoracic, lumbar and sacral regions) of the spinal cord was done on whole mount spinal cord immunolabeled with anti-cleaved caspase-3 antibody (n = 3). Data in (B) and (C) are plotted in box-and-whisker format. Values in (D) represent means ± standard deviation (S.D). Scale bar, 100 µm, (*P<0.05 and **P<0.01).
Figure 4. Role of trophic factors in motoneuron survival of _Necdin_-deficient E12.5 embryos.
(A) Dissections of the lumbar spinal cord regions were performed at E12.5 and the explants were cultured in the presence or in the absence of NTFs for 2 days. Cryostat transverse sections of explants were then immunolabeled using Islet-1/-2 antibodies (green) in order to define the number of surviving motoneurons per explant. (B) In the presence of NTFs (+NTFs), the number of Islet-1/-2 positive cells is not significantly higher in mutant (Necdin+/−) explants (n = 5) compared with wildtype (Necdin+/+) explants (n = 5). (C) In the absence of NTFs (−NTFs), a significant 35% decrease of Islet-1/-2 positive cells is observed in _Necdin-_deficient (Necdin+/−) explants (n = 8) compared to wildtype (Necdin+/+) explants (n = 8), suggesting that the increase of cell death in _Necdin-_deficient motoneurons is not dependant on NTFs. Scale bar: 100 µm.
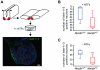
Figure 5. TNFR1 and Necdin are coexpressed in lumbar motoneurons at E12.5.
(A–C) Immunohistochemistry analysis revealing the expression of the death receptors Unc5 (A) or TNFR1 (B and C), on different transversal sections corresponding to the brachial, thoracic and lumbar levels of E12.5 spinal cord. (A) Unc5 (green) is expressed in the brachial ventral horn of the spinal cord. Its expression is fainter in the thoracic ventral horn and is not detected in the lumbar ventral horn. (B) Necdin (red) and TNFR1 (green) are coexpressed in motoneurons of the lumbar region. (C) Higher magnification of (B) showing the colocalization of Necdin with TNFR1. Scale bar: A and B, 100 µm; C, 250 µm.
Figure 6. Necdin deficiency decreases motoneuron survival in absence of NTF but does not alter axonal growth.
(A) Embryonic motoneurons isolated from _Necdin+/+_and Necdin+/− mice were cultured in the presence (+NTFs) or in the absence (−NTFs) of a cocktail of NTFs. After 24 hours, surviving motoneurons were counted and for each genotype survival is expressed as the percentage of the number of motoneurons surviving in the presence of NTF. In absence of NTFs a significant decrease of surviving motoneurons is observed in mutant compared to wildtype motoneurons. (B) When cultured for 3 days in the presence of NTFs Tau immunolabeling reveals a similar morphology between both genotypes. (C) When measuring, axonal length no difference is detected between mutant (Necdin+/−) and wildtype (Necdin+/+) motoneurons. Values are means ± S.D (in (A) or ± standard error of the mean in (B).
Figure 7. Increase of motoneuron cell death in absence of Necdin is linked to the TNFR1 pathway.
(A) When cultured in presence of NTFs Necdin wildtype (Necdin+/+) and mutant (Necdin+/−) motoneurons are equally sensitive to death triggered by TNF alpha. Embryonic mutant and wildtype motoneurons were cultured in presence of NTFs for 24 hours and then treated or not with TNFα. After 48 hours of treatment motoneuron survival was assessed and expressed relative to non-treated cells. (B) In the absence of NTFs, TNFR1-Fc does not rescue cell death observed in wildtype motoneurons, but it restores the level of cell death in Necdin deficient motoneurons to the level observed in wild type cells. Wildtype or mutant motoneurons were cultured in presence (+) or absence (−) of NTFs and treated or not with 100 ng/ml of TNFR1-Fc 1 hours after seeding. Motoneuron survival was determined 24 hours after treatment and expressed as the percentage of the number of motoneurons surviving in the presence of NTF under same conditions. These results suggest that the increase of cell death observed in _Necdin_-deficient motoneurons is linked to the TNFα/TNFR1 pathway. Histograms show mean values ± S.D of triplicate wells in at least three independent experiments.
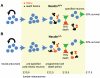
Figure 8. A proposed model for the anti-apoptotic role of Necdin in developing motoneurons.
During embryogenesis, the development of motoneurons starts by a proliferation step in which Necdin is not expressed. These neuronal precursors differentiate into post-mitotic neurons and start to express Necdin (E10.5). These pre-specified motoneurons become sensitive to external death factors and in a normal situation (A) half of them will die and half of them will survive thanks to NTFs. In absence of Necdin (B), motoneurons are more sensitive to the TNFα death factor and an increase in cell death is observed.
Similar articles
- Necdin: A purposive integrator of molecular interaction networks for mammalian neuron vitality.
Yoshikawa K. Yoshikawa K. Genes Cells. 2021 Sep;26(9):641-683. doi: 10.1111/gtc.12884. Epub 2021 Aug 2. Genes Cells. 2021. PMID: 34338396 Free PMC article. Review. - Sensory defects in Necdin deficient mice result from a loss of sensory neurons correlated within an increase of developmental programmed cell death.
Andrieu D, Meziane H, Marly F, Angelats C, Fernandez PA, Muscatelli F. Andrieu D, et al. BMC Dev Biol. 2006 Nov 20;6:56. doi: 10.1186/1471-213X-6-56. BMC Dev Biol. 2006. PMID: 17116257 Free PMC article. - Prader-Willi syndrome protein necdin regulates the nucleocytoplasmic distribution and dopaminergic neuron development.
Li X, Zhang Y, Hu Y, Tang X, Gong Z, Lu RB, Li JD. Li X, et al. Sci Rep. 2024 Dec 30;14(1):31605. doi: 10.1038/s41598-024-76981-y. Sci Rep. 2024. PMID: 39738217 Free PMC article. - Necdin, a Prader-Willi syndrome candidate gene, regulates gonadotropin-releasing hormone neurons during development.
Miller NL, Wevrick R, Mellon PL. Miller NL, et al. Hum Mol Genet. 2009 Jan 15;18(2):248-60. doi: 10.1093/hmg/ddn344. Epub 2008 Oct 17. Hum Mol Genet. 2009. PMID: 18930956 Free PMC article. - Developmental motoneuron cell death and neurotrophic factors.
Sendtner M, Pei G, Beck M, Schweizer U, Wiese S. Sendtner M, et al. Cell Tissue Res. 2000 Jul;301(1):71-84. doi: 10.1007/s004410000217. Cell Tissue Res. 2000. PMID: 10928282 Review.
Cited by
- Preclinical Testing in Translational Animal Models of Prader-Willi Syndrome: Overview and Gap Analysis.
Carias KV, Wevrick R. Carias KV, et al. Mol Ther Methods Clin Dev. 2019 Mar 14;13:344-358. doi: 10.1016/j.omtm.2019.03.001. eCollection 2019 Jun 14. Mol Ther Methods Clin Dev. 2019. PMID: 30989085 Free PMC article. Review. - Age-dependent modulation of cortical transcriptomes in spinal cord injury and repair.
Jaerve A, Kruse F, Malik K, Hartung HP, Müller HW. Jaerve A, et al. PLoS One. 2012;7(12):e49812. doi: 10.1371/journal.pone.0049812. Epub 2012 Dec 7. PLoS One. 2012. PMID: 23236355 Free PMC article. - Antagonistic interplay between necdin and Bmi1 controls proliferation of neural precursor cells in the embryonic mouse neocortex.
Minamide R, Fujiwara K, Hasegawa K, Yoshikawa K. Minamide R, et al. PLoS One. 2014 Jan 2;9(1):e84460. doi: 10.1371/journal.pone.0084460. eCollection 2014. PLoS One. 2014. PMID: 24392139 Free PMC article. - Necdin: A purposive integrator of molecular interaction networks for mammalian neuron vitality.
Yoshikawa K. Yoshikawa K. Genes Cells. 2021 Sep;26(9):641-683. doi: 10.1111/gtc.12884. Epub 2021 Aug 2. Genes Cells. 2021. PMID: 34338396 Free PMC article. Review. - Necdin promotes ubiquitin-dependent degradation of PIAS1 SUMO E3 ligase.
Gur I, Fujiwara K, Hasegawa K, Yoshikawa K. Gur I, et al. PLoS One. 2014 Jun 9;9(6):e99503. doi: 10.1371/journal.pone.0099503. eCollection 2014. PLoS One. 2014. PMID: 24911587 Free PMC article.
References
- Sutcliffe JS, Han M, Christian SL, Ledbetter DH. Neuronally-expressed necdin gene: an imprinted candidate gene in Prader- Willi syndrome [letter]. Lancet. 1997;350:1520–1521. - PubMed
- Jay P, Rougeulle C, Massacrier A, Moncla A, Mattei MG, et al. The human necdin gene, NDN, is maternally imprinted and located in the Prader-Willi syndrome chromosomal region. Nat Genet. 1997;17:357–361. - PubMed
- MacDonald HR, Wevrick R. The necdin gene is deleted in Prader-Willi syndrome and is imprinted in human and mouse. Hum Mol Genet. 1997;6:1873–1878. - PubMed
- Gerard M, Hernandez L, Wevrick R, Stewart CL. Disruption of the mouse necdin gene results in early post-natal lethality. Nat Genet. 1999;23:199–202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases