Neutralizing antibodies against AAV serotypes 1, 2, 6, and 9 in sera of commonly used animal models - PubMed (original) (raw)
Neutralizing antibodies against AAV serotypes 1, 2, 6, and 9 in sera of commonly used animal models
Kleopatra Rapti et al. Mol Ther. 2012 Jan.
Abstract
Adeno-associated virus (AAV)-based vectors are promising gene delivery vehicles for human gene transfer. One significant obstacle to AAV-based gene therapy is the high prevalence of neutralizing antibodies in humans. Until now, it was thought that, except for nonhuman primates, pre-existing neutralizing antibodies are not a problem in small or large animal models for gene therapy. Here, we demonstrate that sera of several animal models of cardiovascular diseases harbor pre-existing antibodies against the cardiotropic AAV serotypes AAV1, AAV6, and AAV9 and against AAV2. The neutralizing antibody titers vary widely both between species and between serotypes. Of all species tested, rats displayed the lowest levels of neutralizing antibodies. Surprisingly, naive mice obtained directly from commercial vendors harbored neutralizing antibodies. Of the large animal models tested, the neutralization of AAV6 transduction by dog sera was especially pronounced. Sera of sheep and rabbits showed modest neutralization of AAV transduction whereas porcine sera strongly inhibited transduction by all AAV serotypes and displayed the largest variation between individual animals. Importantly, neutralizing antibody titers as low as 1/4 completely prevented in vivo transduction by AAV9 in rats. Our results suggest that prescreening of animals for neutralizing antibodies will be important for future gene transfer experiments in these animal models.
Figures
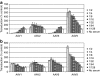
Figure 1
Pooled mouse serum inhibits transduction by adeno-associated virus (AAV) serotypes 1 and 6, but not by serotypes 2 and 9. (a) Pooled mouse serum inhibits AAV transduction. Twofold dilutions of pooled mouse serum were incubated with constant amounts of AAV serotypes 1, 2, 6, and 9 and tested for neutralization of transduction using the in vitro assay described in Materials and Methods. Transduction is expressed as mean percent transduction of no-serum control samples ± SD. (b) Depletion of immunoglobulins drastically reduces the inhibition of AAV transduction by mouse serum. Pooled mouse serum was depleted of immunoglobulins by incubation with protein G resin and a rabbit anti-mouse immunoglobulin M (IgM) antibody. The effect of twofold dilutions of the precleared serum on AAV transduction was assayed as described in (a).
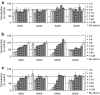
Figure 2
Mouse immunoglobulins M (IgMs) inhibit transduction by adeno-associated virus (AAV) serotypes. (a) Mouse IgMs but no IgGs inhibit AAV transduction. Purified mouse IgG was diluted in Dulbecco's modified Eagle's medium (DMEM) to a concentration found in mouse serum. Twofold dilutions were incubated with constant amounts of AAV serotypes 1, 2, 6, and tested for neutralization of transduction using the in vitro assay described in Materials and Methods. Transduction is expressed as mean percent transduction of no IgG control ± SD. (b) As in (a) but with mouse IgM. (c) As in (b) but with mouse IgG and IgM combined.
Figure 3
Even rats with low in vitro neutralizing titers show strongly reduced in vivo transduction. Rats were immunized with different amounts of empty adeno-associated virus serotype 9 (AAV9) capsids or were left untreated (rats 7, 8, and 15). Five or seven days after immunization the rats were injected with AAV9scEGFP and sacrificed 1 month after vector injection. Protein levels and AAV viral genomes in the heart were quantified as described in Materials and Methods. (a) Western blot against enhanced green fluorescent protein (EGFP) and GAPDH. (b) Quantification of EGFP/GAPDH expression in (a). (c) Viral vector genomes in the heart measured by quantitative PCR (qPCR) ; β actin was used as an internal control. Values in (c) are expressed relative to the levels found in the nonimmunized rat 7 (mean ± SD). The asterisk indicates a nonspecific band recognized by the anti-GFP antibody.
Similar articles
- Pre-existing antibodies to candidate gene therapy vectors (adeno-associated vector serotypes) in domestic cats.
Li P, Boenzli E, Hofmann-Lehmann R, Helfer-Hungerbuehler AK. Li P, et al. PLoS One. 2019 Mar 21;14(3):e0212811. doi: 10.1371/journal.pone.0212811. eCollection 2019. PLoS One. 2019. PMID: 30897117 Free PMC article. - Seroprevalence of binding and neutralizing antibodies against 18 adeno-associated virus types in patients with neuromuscular disorders.
Wang X, Klann PJ, Wiedtke E, Sano Y, Fischer N, Schiller L, Elfert A, Güttsches AK, Weyen U, Grimm D, Vorgerd M, Bayer W. Wang X, et al. Front Immunol. 2024 Sep 27;15:1450858. doi: 10.3389/fimmu.2024.1450858. eCollection 2024. Front Immunol. 2024. PMID: 39399494 Free PMC article. - Prevalence of Pre-Existing Neutralizing Antibodies Against Adeno-Associated Virus Serotypes 1, 2, 5, 6, 8, and 9 in Sera of Different Pig Strains.
Dai Y, Kavita U, Lampen MH, Gielen S, Banks G, Levesque P, Kozhich A, Pillutla R, Zhang Y, Jawa V, Adam L. Dai Y, et al. Hum Gene Ther. 2022 Apr;33(7-8):451-459. doi: 10.1089/hum.2021.213. Hum Gene Ther. 2022. PMID: 34913759 - Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy.
Louis Jeune V, Joergensen JA, Hajjar RJ, Weber T. Louis Jeune V, et al. Hum Gene Ther Methods. 2013 Apr;24(2):59-67. doi: 10.1089/hgtb.2012.243. Epub 2013 Apr 3. Hum Gene Ther Methods. 2013. PMID: 23442094 Free PMC article. Review. - Anti-AAV Antibodies in AAV Gene Therapy: Current Challenges and Possible Solutions.
Weber T. Weber T. Front Immunol. 2021 Mar 17;12:658399. doi: 10.3389/fimmu.2021.658399. eCollection 2021. Front Immunol. 2021. PMID: 33815421 Free PMC article. Review.
Cited by
- Drug Delivery to the Brain: Recent Advances and Unmet Challenges.
Bhunia S, Kolishetti N, Vashist A, Yndart Arias A, Brooks D, Nair M. Bhunia S, et al. Pharmaceutics. 2023 Nov 23;15(12):2658. doi: 10.3390/pharmaceutics15122658. Pharmaceutics. 2023. PMID: 38139999 Free PMC article. Review. - AAV2-VEGF-B gene therapy failed to induce angiogenesis in ischemic porcine myocardium due to inflammatory responses.
Korpela H, Lampela J, Airaksinen J, Järveläinen N, Siimes S, Valli K, Nieminen T, Turunen M, Grönman M, Saraste A, Knuuti J, Hakulinen M, Poutiainen P, Kärjä V, Nurro J, Ylä-Herttuala S. Korpela H, et al. Gene Ther. 2022 Nov;29(10-11):643-652. doi: 10.1038/s41434-022-00322-9. Epub 2022 Feb 7. Gene Ther. 2022. PMID: 35132204 Free PMC article. - Membrane fusion FerA domains enhance adeno-associated virus vector transduction.
Zhang X, Anthony B, Chai Z, Lee Dobbins A, Sutton RB, Li C. Zhang X, et al. Biomaterials. 2020 May;241:119906. doi: 10.1016/j.biomaterials.2020.119906. Epub 2020 Feb 21. Biomaterials. 2020. PMID: 32114218 Free PMC article. - Optogenetics for light control of biological systems.
Emiliani V, Entcheva E, Hedrich R, Hegemann P, Konrad KR, Lüscher C, Mahn M, Pan ZH, Sims RR, Vierock J, Yizhar O. Emiliani V, et al. Nat Rev Methods Primers. 2022;2:55. doi: 10.1038/s43586-022-00136-4. Epub 2022 Jul 21. Nat Rev Methods Primers. 2022. PMID: 37933248 Free PMC article. - The AAV vector toolkit: poised at the clinical crossroads.
Asokan A, Schaffer DV, Samulski RJ. Asokan A, et al. Mol Ther. 2012 Apr;20(4):699-708. doi: 10.1038/mt.2011.287. Epub 2012 Jan 24. Mol Ther. 2012. PMID: 22273577 Free PMC article. Review.
References
- Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF.et al. (2010Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors Hum Gene Ther 21704–712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 HL100396-01/HL/NHLBI NIH HHS/United States
- HL088434/HL/NHLBI NIH HHS/United States
- R01 GM082946-03S1/GM/NIGMS NIH HHS/United States
- HL007824/HL/NHLBI NIH HHS/United States
- R01 GM082946-03/GM/NIGMS NIH HHS/United States
- R01 GM082946-01/GM/NIGMS NIH HHS/United States
- R01 HL088434/HL/NHLBI NIH HHS/United States
- T32 HL007824-13/HL/NHLBI NIH HHS/United States
- R01 HL097088-01A2/HL/NHLBI NIH HHS/United States
- T32 HL007824/HL/NHLBI NIH HHS/United States
- R01 HL088434-02/HL/NHLBI NIH HHS/United States
- R01 GM082946/GM/NIGMS NIH HHS/United States
- T32 HL007824-12/HL/NHLBI NIH HHS/United States
- GM082946/GM/NIGMS NIH HHS/United States
- P20 HL100396-02/HL/NHLBI NIH HHS/United States
- R01 GM082946-04/GM/NIGMS NIH HHS/United States
- R01 HL097088/HL/NHLBI NIH HHS/United States
- R01 HL088434-01A2/HL/NHLBI NIH HHS/United States
- HL100396/HL/NHLBI NIH HHS/United States
- HL097088/HL/NHLBI NIH HHS/United States
- P20 HL100396/HL/NHLBI NIH HHS/United States
- R01 GM082946-05/GM/NIGMS NIH HHS/United States
- R01 GM082946-02/GM/NIGMS NIH HHS/United States
- R01 HL097088-02/HL/NHLBI NIH HHS/United States
- R01 HL097088-03/HL/NHLBI NIH HHS/United States
- R01 HL088434-03/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources