Functional duality of astrocytes in myelination - PubMed (original) (raw)
Comparative Study
Functional duality of astrocytes in myelination
Besma Nash et al. J Neurosci. 2011.
Abstract
Astrocytes undergo major phenotypic changes in response to injury and disease that directly influence repair in the CNS, but the mechanisms involved are poorly understood. Previously, we have shown that neurosphere-derived rat astrocytes plated on poly-L-lysine (PLL-astrocytes) support myelination in dissociated rat spinal cord cultures (myelinating cultures). It is hypothesized that astrocyte reactivity can affect myelination, so we have exploited this culture system to ascertain how two distinct astrocyte phenotypes influence myelination. Astrocytes plated on tenascin C (TnC-astrocytes), a method to induce quiescence, resulted in less myelinated fibers in the myelinating cultures when compared with PLL-astrocytes. In contrast, treatment of myelinating cultures plated on PLL-astrocytes with ciliary neurotrophic factor (CNTF), a cytokine known to induce an activated astrocyte phenotype, promoted myelination. CNTF could also reverse the effect of quiescent astrocytes on myelination. A combination of microarray gene expression analysis and quantitative real-time PCR identified CXCL10 as a potential candidate for the reduction in myelination in cultures on TnC-astrocytes. The effect of TnC-astrocytes on myelination was eliminated by neutralizing CXCL10 antibodies. Conversely, CXCL10 protein inhibited myelination on PLL-astrocytes. Furthermore, CXCL10 treatment of purified oligodendrocyte precursor cells did not affect proliferation, differentiation, or process extension compared with untreated controls, suggesting a role in glial/axonal ensheathment. These data demonstrate a direct correlation of astrocyte phenotypes with their ability to support myelination. This observation has important implications with respect to the development of therapeutic strategies to promote CNS remyelination in demyelinating diseases.
Figures
Figure 1.
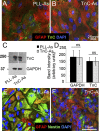
Biological properties of astrocytes plated on TnC compared with astrocytes plated on PLL. A, B, PLL-As (A) or TnC-As (B) were fixed and immunostained for GFAP, TnC, and DAPI. C, D, Sister astrocyte cultures were lysed and immunoblotted for TnC and GAPDH expression (C), and the intensity of the Western blots was quantified from three experiments (D). TnC-As did not express higher levels of TnC compared with PLL-As. E, F, PLL-As and TnC-As were immunolabeled with anti-GFAP, nestin, and DAPI.
Figure 2.
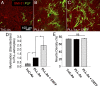
Myelination was reduced on quiescent astrocytes. A–C, Myelinating cultures were plated on PLL-As (A) or TnC-As (B). PLL-As were treated with 2 ng/ml CNTF from day 12 onward (C). D, Myelination was significantly higher on PLL-As compared with those plated on TnC-As. Treatment of cultures on PLL-As with CNTF resulted in a significant increase in myelination compared with untreated PLL-As. E, Neurite density was similar in all conditions. *p < 0.05.
Figure 3.
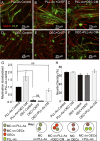
CNTF does not have a promyelinating effect when cultures are plated on a monolayer of OECs. A–F, Myelinating cultures were plated on PLL-As (A–C) or OECs (D–F) and left untreated (A, D) or treated with CNTF (2 ng/m) (B, E). Monolayers of astrocyte (PLL-As) cultures were included in the same dish as myelinating cultures plated on an OEC monolayer to condition the medium supporting the myelinating cultures (F) and, conversely, monolayers of OECs were placed in the same dish as myelinated cultures plated on a astrocyte monolayer (C). G, Cultures plated on a monolayer of OEC do not show the same increase in myelination with the addition of exogenous CNTF. Quantification of myelination showing a significant increase in cultures plated on OEC monolayers and conditioned by PLL-As. H, Neurite density as a percentage of SMI-31 immunoreactivity for conditions shown in A–F (*p < 0.05; ns, _p_ > 0.05). MC, Myelinating cultures.
Figure 4.
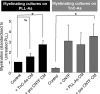
Activation of astrocytes by CNTF increases myelination. Myelinating cultures were plated onto PLL-As (black bars) or TnC-As (gray bars). CNTF treatment increased myelination in all cultures. When TnC-As were used to condition cultures plated on PLL-As, myelination was not affected, suggesting that the TnC effect is overcome by PLL-As. Conversely, cultures plated on TnC-As conditioned by coverslips of PLL-As promoted myelination. PLL-As pretreated with CNTF, washed, and then added to cultures resulted in an increase in myelination, suggesting the CNTF activates the astrocyte directly (*p < 0.05; ns, _p_ > 0.05).
Figure 5.
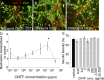
Titration of CNTF produced variable effects on myelination. A–C, E15.5 myelinating cultures were plated onto a confluent monolayer of astrocytes (PLL-As) and cultured for 26–28 d. On day 12, cultures were left untreated or treated with varying concentrations of CNTF (0.02 pg–20 ng/ml). Representative figures of low, intermediate, and high concentrations in which low and high concentrations had no effect on myelination compared with control. D, Graphical representation of the dose-dependent effect on myelination by CNTF (*p < 0.05 vs control). Myelinated axons were calculated and standardized to controls for each respective experiment; the dotted line represents the standardized level for nontreated cultures. E, Neurite density (assessed by SMI-31 reactivity) was unchanged at each concentration.
Figure 6.
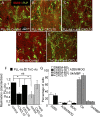
Candidate genes identified by microarray are verified via qRT-PCR. A, A four-way Venn diagram in which each rectangle (A, B, C, and D) represents a pairwise comparison between TnC-astrocytes after 4 h (TnC 4 h) and one of the other four conditions: PLL-astrocytes after 4 h (PLL 4 h) and 24 h (PLL 24 h), PLL-astrocytes treated with CNTF after 4 h (PLL+CNTF 4 h) and 24 h (PLL+CNTF 24 h). Each of the 15 segments indicates one particular intersection, e.g., “A_C_” contains the number of probes (genes) significantly different for the A (TnC 4 h vs PLL 4 h) and C (TnC 4 h vs PLL 24 h) comparisons but not the B (TnC 4 h vs PLL+CNTF 4 h) and D (TnC 4 h vs PLL+CNTF 24 h) comparisons. Numbers shown indicate the number of significant genes (FDR < 0.05, n = 3) for each combination. B, Heatmap with dendrogram of the 15 probes significantly different in all four comparisons involving TnC-astrocytes after 4 h (“ABCD” in Venn diagram). Green is low expression, red is high, black is intermediate; expression is standardized within each gene, and within-group median expression is shown. Dendrogram uses complete linkage and Euclidean distance. C, qRT-PCR, relative quantification (RQ) results for CXLCL10, CTGF, and THBS4. CXCL10 mRNA was significantly increased in TnC-astrocytes when compared with astrocytes treated with CNTF (Fig. 7_B_) (*p < 0.05, n = 3).
Figure 7.
CXCL10 has a negative effect on myelination. A–E, Myelinating cultures were plated onto PLL-As (A–C) or TnC-As (D, E) and left untreated (A, D), or treated with CXCL10 neutralizing antibody (2 μg/ml, C, E). F, Treatment of TnC-As cultures with CXCL10 neutralizing antibody led to an increase in myelination compared with untreated cultures. Myelinating cultures plated on PLL-As also received 10 ng/ml CXCL10 on day 12 and onward (B, F), which resulted in a reduction in myelination (*p < 0.05). G, Purified OPCs were double-immunolabeled with the O4 antibody and anti-MBP, in addition to A2B5 and anti-MOG, to assess differentiation after CXCL10 treatment for 7 d, followed by staining on day 8 (n = 4). No differences in the percentage of differentiated cells were observed with CXCL10 treatment.
Similar articles
- Astrocyte phenotypes and their relationship to myelination.
Nash B, Ioannidou K, Barnett SC. Nash B, et al. J Anat. 2011 Jul;219(1):44-52. doi: 10.1111/j.1469-7580.2010.01330.x. Epub 2010 Dec 24. J Anat. 2011. PMID: 21496013 Free PMC article. - Astrocytes, but not olfactory ensheathing cells or Schwann cells, promote myelination of CNS axons in vitro.
Sorensen A, Moffat K, Thomson C, Barnett SC. Sorensen A, et al. Glia. 2008 May;56(7):750-63. doi: 10.1002/glia.20650. Glia. 2008. PMID: 18293402 - Ciliary neurotrophic factor activates spinal cord astrocytes, stimulating their production and release of fibroblast growth factor-2, to increase motor neuron survival.
Albrecht PJ, Dahl JP, Stoltzfus OK, Levenson R, Levison SW. Albrecht PJ, et al. Exp Neurol. 2002 Jan;173(1):46-62. doi: 10.1006/exnr.2001.7834. Exp Neurol. 2002. PMID: 11771938 - Engineering biomaterial microenvironments to promote myelination in the central nervous system.
Unal DB, Caliari SR, Lampe KJ. Unal DB, et al. Brain Res Bull. 2019 Oct;152:159-174. doi: 10.1016/j.brainresbull.2019.07.013. Epub 2019 Jul 12. Brain Res Bull. 2019. PMID: 31306690 Review. - Remyelination within the CNS: do schwann cells pave the way for oligodendrocytes?
Jasmin L, Ohara PT. Jasmin L, et al. Neuroscientist. 2002 Jun;8(3):198-203. doi: 10.1177/1073858402008003005. Neuroscientist. 2002. PMID: 12061499 Review.
Cited by
- Myelination of rodent hippocampal neurons in culture.
Gardner A, Jukkola P, Gu C. Gardner A, et al. Nat Protoc. 2012 Oct;7(10):1774-82. doi: 10.1038/nprot.2012.100. Epub 2012 Sep 6. Nat Protoc. 2012. PMID: 22955693 Free PMC article. - Extracellular matrix composition determines astrocyte responses to mechanical and inflammatory stimuli.
Johnson KM, Milner R, Crocker SJ. Johnson KM, et al. Neurosci Lett. 2015 Jul 23;600:104-9. doi: 10.1016/j.neulet.2015.06.013. Epub 2015 Jun 9. Neurosci Lett. 2015. PMID: 26067407 Free PMC article. - Heterogeneity of white matter astrocytes in the human brain.
Bugiani M, Plug BC, Man JHK, Breur M, van der Knaap MS. Bugiani M, et al. Acta Neuropathol. 2022 Feb;143(2):159-177. doi: 10.1007/s00401-021-02391-3. Epub 2021 Dec 8. Acta Neuropathol. 2022. PMID: 34878591 Review. - Copper chelation and autoimmunity differentially impact myelin in the hippocampal-prefrontal circuit.
Nickel M, Eid F, Jukkola P, Gu C. Nickel M, et al. J Neuroimmunol. 2019 Sep 15;334:576998. doi: 10.1016/j.jneuroim.2019.576998. Epub 2019 Jun 20. J Neuroimmunol. 2019. PMID: 31254928 Free PMC article. - Human Primary Astrocytes Differently Respond to Pro- and Anti-Inflammatory Stimuli.
Szpakowski P, Ksiazek-Winiarek D, Turniak-Kusy M, Pacan I, Glabinski A. Szpakowski P, et al. Biomedicines. 2022 Jul 22;10(8):1769. doi: 10.3390/biomedicines10081769. Biomedicines. 2022. PMID: 35892669 Free PMC article.
References
- Abney ER, Williams BP, Raff MC. Tracing the development of oligodendrocytes from precursor cells using monoclonal antibodies, fluorescence-activated cell sorting, and cell culture. Dev Biol. 1983;100:166–171. - PubMed
- Albrecht PJ, Murtie JC, Ness JK, Redwine JM, Enterline JR, Armstrong RC, Levison SW. Astrocytes produce CNTF during the remyelination phase of viral-induced spinal cord demyelination to stimulate FGF-2 production. Neurobiol Dis. 2003;13:89–101. - PubMed
- Albrecht PJ, Enterline JC, Cromer J, Levison SW. CNTF-activated astrocytes release a soluble trophic activity for oligodendrocyte progenitors. Neurochem Res. 2007;32:263–271. - PubMed
- Alexander CL, Fitzgerald UF, Barnett SC. Identification of growth factors that promote long-term proliferation of olfactory ensheathing cells and modulate their antigenic phenotype. Glia. 2002;37:349–364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous