p16(INK4a) expression and breast cancer risk in women with atypical hyperplasia - PubMed (original) (raw)
p16(INK4a) expression and breast cancer risk in women with atypical hyperplasia
Derek C Radisky et al. Cancer Prev Res (Phila). 2011 Dec.
Abstract
p16, a nuclear protein encoded by the p16(INK4a) gene, is a regulator of cell-cycle regulation. Previous studies have shown that expression of p16 in tissue biopsies of patients with ductal carcinoma in situ (DCIS) is associated with increased risk of breast cancer, particularly when considered in combination with other markers such as Ki-67 and COX-2. Here, we evaluated how expression of p16 in breast tissue biopsies of women with atypical hyperplasia (AH), a putative precursor lesion to DCIS, is associated with subsequent development of cancer. p16 expression was assessed by immunohistochemistry in archival sections from 233 women with AH diagnosed at the Mayo Clinic. p16 expression in the atypical lesions was scored by percentage of positive cells and intensity of staining. We also studied coexpression of p16, with Ki-67 and COX-2, biomarkers of progression in AH. Risk factor and follow-up data were obtained via study questionnaire and medical records. Forty-seven patients (20%) developed breast cancer with a median follow-up of 14.5 years. Staining of p16 was increased in older patients relative to younger patients (P = 0.0025). Although risk of developing breast cancer was not associated with increased p16 expression, joint overexpression of Ki-67 and COX-2 was found to convey stronger risk of breast cancer in the first 10 years after diagnosis as compared with one negative marker (P < 0.01). However, the addition of p16 levels did not strengthen this association. p16 overexpression, either alone or in combination with COX-2 and Ki-67, does not significantly stratify breast cancer risk in women with AH.
2011 AACR
Figures
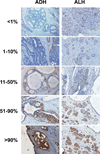
Figure 1
Staining levels of p16 in AH. Samples of atypical ductal hyperplasia (ADH, left column) and atypical lobular hyperplasia (ALH, right column), showing fractional staining levels of <1% cells staining positively for p16 (top row), 1–10% p16+ cells (second row), 11–50% p16+ cells (third row), 51–90% p16+ cells (fourth row), and >90% p16+ cells (bottom row).
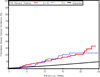
Figure 2
Expression of p16 is not related to cumulative breast cancer incidence or time to breast cancer for women with AH. Observed and expected events are cumulated after accounting for death as a competing risk and are plotted as a function of follow-up interval and stratified by p16 expression levels. Red line, 0–10% cells staining for p16; blue line, 11+% cells staining for p16; black line, expected breast cancer incidence according to Iowa Surveillance, Epidemiology, and End Results survey.
Comment in
- Molecular biomarkers of risk in premalignancy and breast cancer prevention.
Allred DC. Allred DC. Cancer Prev Res (Phila). 2011 Dec;4(12):1947-52. doi: 10.1158/1940-6207.CAPR-11-0478. Cancer Prev Res (Phila). 2011. PMID: 22144468
Similar articles
- COX-2, p16 and Ki67 expression in DCIS, microinvasive and early invasive breast carcinoma with extensive intraductal component.
Bartova M, Ondrias F, Muy-Kheng T, Kastner M, Singer Ch, Pohlodek K. Bartova M, et al. Bratisl Lek Listy. 2014;115(7):445-51. doi: 10.4149/bll_2014_088. Bratisl Lek Listy. 2014. PMID: 25077370 - Biomarker expression and risk of subsequent tumors after initial ductal carcinoma in situ diagnosis.
Kerlikowske K, Molinaro AM, Gauthier ML, Berman HK, Waldman F, Bennington J, Sanchez H, Jimenez C, Stewart K, Chew K, Ljung BM, Tlsty TD. Kerlikowske K, et al. J Natl Cancer Inst. 2010 May 5;102(9):627-37. doi: 10.1093/jnci/djq101. Epub 2010 Apr 28. J Natl Cancer Inst. 2010. PMID: 20427430 Free PMC article. - Ki67: a time-varying biomarker of risk of breast cancer in atypical hyperplasia.
Santisteban M, Reynolds C, Barr Fritcher EG, Frost MH, Vierkant RA, Anderson SS, Degnim AC, Visscher DW, Pankratz VS, Hartmann LC. Santisteban M, et al. Breast Cancer Res Treat. 2010 Jun;121(2):431-7. doi: 10.1007/s10549-009-0534-7. Epub 2009 Sep 23. Breast Cancer Res Treat. 2010. PMID: 19774459 Free PMC article. - [Atypical adenomatous hyperplasia of lung: clinicopathologic study of 8 cases and review of literature].
Huo Z, Liu HR, Wan JW. Huo Z, et al. Zhonghua Bing Li Xue Za Zhi. 2007 May;36(5):292-6. Zhonghua Bing Li Xue Za Zhi. 2007. PMID: 17706134 Review. Chinese. - Atypical lobular hyperplasia and lobular carcinoma in situ: surgical and molecular pathology.
Lishman SC, Lakhani SR. Lishman SC, et al. Histopathology. 1999 Sep;35(3):195-200. doi: 10.1046/j.1365-2559.1999.00815.x. Histopathology. 1999. PMID: 10469210 Review.
Cited by
- P16 Expression in Human Breast Carcinoma and its Relationship to Clinicopathological Parameters.
Mohammadizadeh F, Nasri F. Mohammadizadeh F, et al. Adv Biomed Res. 2023 Jun 28;12:154. doi: 10.4103/abr.abr_180_22. eCollection 2023. Adv Biomed Res. 2023. PMID: 37564443 Free PMC article. - Immune cells are increased in normal breast tissues of BRCA1/2 mutation carriers.
Ogony J, Hoskin TL, Stallings-Mann M, Winham S, Brahmbhatt R, Arshad MA, Kannan N, Peña A, Allers T, Brown A, Sherman ME, Visscher DW, Knutson KL, Radisky DC, Degnim AC. Ogony J, et al. Breast Cancer Res Treat. 2023 Jan;197(2):277-285. doi: 10.1007/s10549-022-06786-y. Epub 2022 Nov 16. Breast Cancer Res Treat. 2023. PMID: 36380012 Free PMC article. - Revisiting Epithelial Carcinogenesis.
Méndez-López LF. Méndez-López LF. Int J Mol Sci. 2022 Jul 4;23(13):7437. doi: 10.3390/ijms23137437. Int J Mol Sci. 2022. PMID: 35806442 Free PMC article. - Investigation of p16 protein expression and its association with histopathologic parameters in breast cancer.
Naji-Haddadi S, Elieh-Ali-Komi D, Aghayan S, Asghari R, Rasouli J. Naji-Haddadi S, et al. Mol Biol Res Commun. 2021 Dec;10(4):165-170. doi: 10.22099/mbrc.2021.41691.1671. Mol Biol Res Commun. 2021. PMID: 35097138 Free PMC article. - Gene expression signature of atypical breast hyperplasia and regulation by SFRP1.
Gregory KJ, Roberts AL, Conlon EM, Mayfield JA, Hagen MJ, Crisi GM, Bentley BA, Kane JJ, Makari-Judson G, Mason HS, Yu J, Zhu LJ, Simin K, Johnson JPS, Khan A, Schneider BR, Schneider SS, Jerry DJ. Gregory KJ, et al. Breast Cancer Res. 2019 Jun 27;21(1):76. doi: 10.1186/s13058-019-1157-5. Breast Cancer Res. 2019. PMID: 31248446 Free PMC article.
References
- Ghosh K, Melton LJ, 3rd, Suman VJ, Grant CS, Sterioff S, Brandt KR, et al. Breast biopsy utilization: a population-based study. Arch Intern Med. 2005;165:1593–1598. - PubMed
- Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, Ghosh K, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353:229–237. - PubMed
- Degnim AC, Visscher DW, Berman HK, Frost MH, Sellers TA, Vierkant RA, et al. Stratification of breast cancer risk in women with atypia: a Mayo cohort study. J Clin Oncol. 2007;25:2671–2677. - PubMed
- Hoogerbrugge N, Bult P, de Widt-Levert LM, Beex LV, Kiemeney LA, Ligtenberg MJL, et al. High prevalence of premalignant lesions in prophylactically removed breasts from women at hereditary risk for breast cancer. Journal of Clinical Oncology. 2003;21:41–45. - PubMed
- Kauff ND, Brogi E, Scheuer L, Pathak DR, Borgen PI, Hudis CA, et al. Epithelial lesions in prophylactic mastectomy specimens from women with BRCA mutations. Cancer. 2003;97:1601–1608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA116201/CA/NCI NIH HHS/United States
- CA132789/CA/NCI NIH HHS/United States
- P50 CA116201-05/CA/NCI NIH HHS/United States
- CA122086/CA/NCI NIH HHS/United States
- P50 CA116201/CA/NCI NIH HHS/United States
- R01 CA132879/CA/NCI NIH HHS/United States
- R01 CA122086-05/CA/NCI NIH HHS/United States
- R01 CA132879-05/CA/NCI NIH HHS/United States
- R01 CA122086/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials